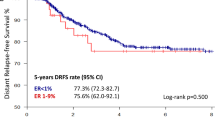
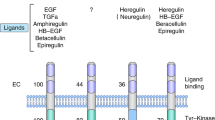
Key Points
-
In addition to phenotypic differences, recently discovered differences in genetic mutations between metastatic and early stage breast cancer mean the advanced state can be considered a different disease
-
New mutations in genes, such as ESR1, provide direct evidence of an evolving disease that likely confers treatment resistance and represent targets for future therapies
-
Other molecular adaptations that can occur in advanced disease also offer promising therapeutic targets using agents such as mTOR, CD4/6 and PI3K inhibitors
-
During metastasis, a switch to oestrogen receptor (ER) or HER2 positive disease is a recognized phenomenon, but the value of altering therapy on the basis of such changes is unclear
-
No biomarkers exist to guide endocrine or biological therapy choices in metastatic disease; however, an appreciation of the clinical evidence of resistance may help
-
Everolimus plus exemestane may be preferable after all lines of single agent endocrine therapy have been employed, and possibly following single-agent chemotherapy
Abstract
Hormone-receptor-positive breast cancer accounts for the majority—up to 80%—of all breast cancers. The evolution of breast cancer from early stage to the metastatic setting leads to increased heterogeneity, the occurrence of new mutations, and the development of treatment resistance representing a great challenge for management decisions. Unfortunately, little data exist to offer guidance in this context, and a reliance on traditional clinical parameters remains when deciding on optimal treatment. In advanced-stage oestrogen receptor-positive (ER+) disease, ongoing issues include the choice between endocrine therapy and chemotherapy, the appropriate sequence of treatment agents, and the incorporation of biological agents, such as everolimus, into the treatment armamentarium. In metastatic disease, repeated biopsies can help to reassess the receptor or genetic mutational status; however, the evidence to support this approach is limited. In this Review, we examine the current evidence that can guide treatment decisions in patients with advanced-stage ER+ breast cancer, discuss how to tackle these therapeutic challenges and provide suggestions for the optimal management of this patient population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Tan, S.-H. & Wolff, A. C. in Diseases of the Breast 4th edn (ed. Harris, J. R., Lippman, M. E., Morrow, M. & Osborne, C. K.) Ch. 73–74 (Lippincott Williams & Wilkins, 2009).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Nielsen, T. O. et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated oestrogen receptor-positive breast cancer. Clin. Cancer Res. 16, 5222–5232 (2010).
Osborne, C. K. & Schiff, R. Mechanisms of endocrine resistance in breast cancer. Ann. Rev. Med. 62, 233–247 (2011).
Hoefnagel, L. D. et al. Discordance in ERα, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann. Oncol. 24, 3017–3023 (2013).
Almendro, V. et al. Inference of tumour evolution during chemotherapy by computational modelling and in situ analysis of cellular diversity for genetic and phenotypic features. Cell Rep. 6, 514–527 (2014).
Tegze, B. et al. Parallel evolution under chemotherapy pressure in 29 breast cancer cell lines results in dissimilar mechanisms of resistance. PLoS ONE 7, e30804 (2012).
Ostrow, S. L., Barshir, R., DeGregori, J., Yeger-Lotem, E. & Hershberg, R. Cancer evolution is associated with pervasive positive selection on globally expressed genes. PLoS Genet. 10, e1004239 (2014).
Toy, W. et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 45, 1439–1445 (2013).
Robinson, D. R. et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 45, 1446–1451 (2013).
Gerlinger, M. et al. Intratumour heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Rivenbark, A. G., O'Connor, S. M. & Coleman, W. B. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am. J. Pathol. 183, 1113–1124 (2013).
Coley, H. M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 34, 378–390 (2008).
Perez, E. A. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast Cancer Res. Treat. 114, 195–201 (2008).
Amiri-Kordestani, L., Basseville, A., Kurdziel, K., Fojo, A. T. & Bates, S. E. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updat. 15, 50–61 (2012).
Ellis, M. J. & Perou, C. M. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 3, 27–34 (2013).
Bachelot, T. et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J. Clin. Oncol. 30, 2718–2724 (2012).
Cardoso, F. et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann. Oncol. 25, 1871–1888 (2014).
Chan, C. M., Martin, L. A., Johnston, S. R., Ali, S. & Dowsett, M. Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J. Steroid Biochem. Mol. Biol. 8, 333–341 (2002).
Jelovac, D., Sabnis, G., Long, B. J., Macedo, L., Goloubeva, O. G. & Brodie, A. M. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 65, 5380–5389 (2005).
Miller, T. W. et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in oestrogen receptor-positive human breast cancer J. Clin. Invest. 120, 2406–2413 (2010).
Turner, N. C. & Reis-Filho, J. S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 13, 178–185 (2012).
Jeselsohn, R. et al. Emergence of constitutively active oestrogen receptor-α mutations in pretreated advanced oestrogen receptor-positive breast cancer. Clin. Cancer Res. 20, 1757–1767 (2014).
Martin, L. A. et al. Enhanced oestrogen receptor (ER) α, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term oestrogen deprivation. J. Biol. Chem. 278, 30458–30468 (2003).
Schiff, R. et al. Cross-talk between oestrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin. Cancer Res. 10, 331S–336S (2004).
Salvatori, L. et al. Oestrogens and selective oestrogen receptor (ER) modulators regulate EGF receptor gene expression through human ER alpha and beta subtypes via an Sp1 site. Oncogene 22, 4875–4881 (2003).
Gutierrez, M. C. et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between oestrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J. Clin. Oncol. 23, 2469–2476 (2005).
Osborne, C. K. et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin. Cancer Res. 17, 1147–1159 (2011).
Robertson, J. F. et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 14, 228–235 (2013).
Migliaccio, I., Di Leo, A. & Malorni, L. Cyclin-dependent kinase 4/6 inhibitors in breast cancer therapy. Curr. Opin. Oncol. 26, 568–575 (2014).
Tokunaga, E. et al. Activation of PI3K/Akt signalling and hormone resistance in breast cancer. Breast Cancer 13, 137–144 (2006).
Miller, T. et al. Resistance to endocrine therapy in oestrogen receptor-positive (ER+) breast cancer is dependent upon phosphatidylinositol-3 kinase (PI3K) signalling [abstract]. Cancer Res. 69 (Suppl.), a403 (2009).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Loi, S. et al. PIK3CA mutations associated with gene signature of low mTORC1 signalling and better outcomes in oestrogen receptor-positive breast cancer. Proc. Natl Acad. Sci. USA 107, 10208–10213 (2010).
Fu, X. et al. Overcoming endocrine resistance due to reduced PTEN levels in oestrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res. 16, 430 (2014).
Dey, N., Leyland-Jones, B. & De, P. MYC-xing it up with PIK3CA mutation and resistance to PI3K inhibitors: summit of two giants in breast cancers. Am. J. Cancer Res. 5, 1–19 (2014).
Treilleux, I. et al. Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann. Oncol. 26, 120–125 (2015).
Krop, I. et al. The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant vs fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer – Part I results [abstract]. San Antonio Breast Cancer Symposium 2014, S2–02 (2014).
Thompson, A. M. et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 12, R92 (2010).
de Dueñas, E. M. et al. Prospective evaluation of the conversion rate in the receptor status between primary breast cancer and metastasis: results from the GEICAM 2009–2003 ConvertHER study. Breast Cancer Res. Treat. 143, 507–515 (2014).
Amir, E. et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat. Rev. 38, 708–714 (2012).
Karlsson, E. et al. Breast cancer during follow-up and progression—a population based cohort on new cancers and changed biology. Eur. J. Cancer 50, 2916–2924 (2014).
Chang, H. J. et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J. Clin. Oncol. 41, 593–599 (2011).
Turner, N. H. & Di Leo, A. HER2 discordance between primary and metastatic breast cancer: assessing the clinical impact. Cancer Treat. Rev. 39, 947–957 (2013).
Fabi, A. et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin. Cancer Res. 17, 2055–2064 (2011).
Tolles, J., Bai, Y., Baquero, M., Harris, L. N., Rimm, D. L. & Molinaro, A. M. Optimal tumour sampling for immunostaining of biomarkers in breast carcinoma. Breast Cancer Res. 13, R51 (2011).
Hortobagyi, G. N. et al. Correlation of molecular alterations with efficacy of everolimus in hormone receptor–positive, HER2-negative advanced breast cancer: results from BOLERO-2 [abstract]. J. Clin. Oncol. 31 (Suppl.), LBA509 (2013).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015).
André, F. et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 15, 267–274 (2014).
Zardavas, D. et al. The AURORA initiative for metastatic breast cancer. Br. J. Cancer 111, 1881–1887 (2014).
Partridge, A. H. et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 32, 3307–3329 (2014).
Mauri, D., Pavlidis, N., Polyzos, N. P. & Ioannidis, J. P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J. Natl Cancer Inst. 98, 1285–1291 (2006).
Robertson, J. F. et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J. Clin. Oncol. 27, 4530–4535 (2009).
Robertson, J. F. et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res. Treat. 136, 503–511 (2012).
Robertson, J. F. et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II 'first' study [abstract]. San Antonio Breast Cancer Symposium S6–04 (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Mehta, R. S. et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N. Engl. J. Med. 367, 435–444 (2012).
Bergh, J. et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J. Clin. Oncol. 30, 1919–1925 (2012).
Di Leo, A. & Malorni, L. Polyendocrine treatment in oestrogen receptor-positive breast cancer: a “FACT” yet to be proven. J. Clin. Oncol. 30, 1897–1900 (2012).
Thürlimann, B. et al. Study Group. Efficacy of tamoxifen following anastrozole ('Arimidex') compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur. J. Cancer 39, 2310–2317 (2003).
Chia, S. et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol. 26, 1664–1670 (2008).
Di Leo, A. et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with oestrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 28, 4594–4600 (2010).
Johnston, S. R. et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 14, 989–998 (2013).
Beck, J. T. et al. Everolimus plus exemestane as first-line therapy in HR+, HER2- advanced breast cancer in BOLERO-2. Breast Cancer Res. Treat. 143, 459–467 (2014).
Ingle, J. N. et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J. Clin. Oncol. 24, 1052–1056 (2006).
deGraffenried, L. A. et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin. Cancer Res. 10 8059–8067 (2004).
Baselga J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 366, 520–529 (2012).
Yardley, D. A. et al. Everolimus plus exemestane in postmenopausalp with HR+ breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 30, 870–884 (2013).
Piccart, M. et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann. Oncol. 25, 2357–2362 (2014).
Wolff, A. C. et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J. Clin. Oncol. 31, 195–202 (2013).
Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with oestrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Hurvitz, S. A. et al. Phase 3, randomized, double-blind, placebo-controlled multicentre trial of daily everolimus plus weekly trastuzumab and paclitaxel as first-line therapy in women with HER2+ advanced breast cancer: BOLERO-1 [abstract]. San Antonio Breast Cancer Symposium S6–01 (2014).
US Department of Health and Human Services. FDA approves Ibrance for postmenopausal women with advanced breast cancer [online], (2015).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD0332991 and associated antitumour activity in human tumour xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Migliaccio, I., Di Leo, A. & Malorni, L. Cyclin-dependent kinase 4/6 inhibitors in breast cancer therapy. Curr. Opin. Oncol. 26, 568–575 (2014).
Bosco, E. E. et al. The retinoblastoma tumour suppressor modifies the therapeutic response of breast cancer. J. Clin. Invest. 117, 218–228 (2007).
Arnold, A. & Papanikolaou, A. Cyclin D1 in breast cancer pathogenesis. J. Clin. Oncol. 23, 4215–4224 (2005).
Stendahl, M. et al. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br. J. Cancer 90, 1942–1948 (2004).
Jirström, K. et al. Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res. 65, 8009–8016 (2005).
Finn, R. S. et al. PD0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal oestrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Yardley, D. A. et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic oestrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 31, 2128–2135 (2013).
Paul, D. et al. Letrozole plus dasatinib improves progression-free survival (PFS) in hormone receptor-positive, HER2-negative postmenopausal metastatic breast cancer (MBC) patients receiving first-line aromatase inhibitor (AI) therapy [abstract]. San Antonio Breast Cancer Symposium S3–07 (2013).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Pagani, O. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 371, 107–118 (2014).
Francis, P. A. et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372, 436–446 (2015).
Acknowledgements
We thank the Sandro Pitigliani Foundation for their generous support.
Author information
Authors and Affiliations
Contributions
C.D.H. and I.M. researched data for article. C.D.H. and A.D.L. wrote the manuscript. C.D.H. and A.D.L. substantially contributed to discussion of content, and all authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.M. is a consultant for AstraZeneca and has a research grant from Pfizer; A.D.L. receives honoraria from AstraZeneca and Novartis, and a research grant from Pfizer. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Hart, C., Migliaccio, I., Malorni, L. et al. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin Oncol 12, 541–552 (2015). https://doi.org/10.1038/nrclinonc.2015.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.99
This article is cited by
-
The value of oral selective estrogen receptor degraders in patients with HR-positive, HER2-negative advanced breast cancer after progression on ≥ 1 line of endocrine therapy: systematic review and meta-analysis
BMC Cancer (2024)
-
A novel bystander effect in tamoxifen treatment: PPIB derived from ER+ cells attenuates ER− cells via endoplasmic reticulum stress-induced apoptosis
Cell Death & Disease (2024)
-
Comparative efficacy and safety of extended adjuvant endocrine therapy for hormone receptor-positive early breast cancer: a Bayesian network meta-analysis
Breast Cancer Research and Treatment (2024)
-
Targeting PEG10 as a novel therapeutic approach to overcome CDK4/6 inhibitor resistance in breast cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
Plasma apolipoprotein M predicts overall survival in metastatic breast cancer patients
Breast Cancer Research and Treatment (2023)