Key Points
-
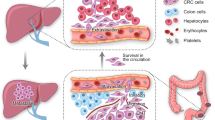
Up to a 20% of patients with colorectal cancer (CRC) present with synchronous hepatic metastases
-
In patients who present without intestinal obstruction or perforation, comprehensive whole-body imaging is required to exclude extrahepatic disease
-
Current evidence indicates a state of equipoise between several different management approaches for the treatment of CRC and synchronous liver metastatic disease, none of which has supportive randomized trial evidence
-
Neoadjuvant systemic chemotherapy is supported by current guidelines and can result in tumour downsizing, enabling some 'unresectable' liver metastases to be surgically removed
-
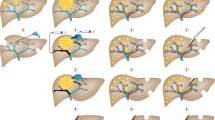
Surgery can take the form of the 'classic' approach (colorectal resection, then interval chemotherapy followed by liver resection), synchronous removal of liver and bowel tumours, or a liver-first approach
-
Clear superiority has not been demonstrated for any of these surgical interventions for CRC with synchronous liver metastases, although the mode of presentation can determine the approach used
Abstract
Up to a fifth of patients with colorectal cancer (CRC) present with synchronous hepatic metastases. In patients with CRC who present without intestinal obstruction or perforation and in whom comprehensive whole-body imaging confirms the absence of extrahepatic disease, evidence indicates a state of equipoise between several different management pathways, none of which has demonstrated superiority. Neoadjuvant systemic chemotherapy is advocated by current guidelines, but must be integrated with surgical management in order to remove the primary tumour and liver metastatic burden. Surgery for CRC with synchronous liver metastases can take a number of forms: the 'classic' approach, involving initial colorectal resection, interval chemotherapy and liver resection as the final step; simultaneous removal of the liver and bowel tumours with neoadjuvant or adjuvant chemotherapy; or a 'liver-first' approach (before or after systemic chemotherapy) with removal of the colorectal tumour as the final procedure. In patients with rectal primary tumours, the liver-first approach can potentially avoid rectal surgery in patients with a complete response to chemoradiotherapy. We overview the importance of precise nomenclature, the influence of clinical presentation on treatment options, and the need for accurate, up-to-date surgical terminology, staging tests and contemporary management options in CRC and synchronous hepatic metastatic disease, with an emphasis on multidisciplinary care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Department of Health and Human Services, Centers for Disease Control and Prevention, National Program of Cancer Registries (NPCR). United States Cancer Statistics (USCS): 1999–2010 Cancer Incidence and Mortality Data [online], (2014).
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49, 1374–1403 (2013).
Leporrier, J. et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br. J. Surg. 93, 465–474 (2006).
Manfredi, S. et al. Epidemiology and management of colorectal liver metastases. Ann. Surg. 244, 254–259 (2006).
Cancer Research UK. Statistics and outlook for bowel cancer [online], (2013).
Tomlinson, J. S. et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J. Clin. Oncol. 25, 4575–4580 (2007).
Jain, V. K., Hawkes, E. A. & Cunningham, D. Integration of biologic agents with cytotoxic chemotherapy in metastatic colorectal cancer. Clin. Colorectal Cancer 10, 245–257 (2011).
Schmoll, H. J. et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 23, 2479–2516 (2012).
Bismuth, H., Castaing, D. & Traynor, O. Surgery for synchronous hepatic metastases of colorectal cancer. Scand. J. Gastroenterol. 149, 144–149 (1988).
Fong, Y. et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 230, 309–318 (1999).
National Institute for Health and Care Excellence. Colorectal Cancer: The diagnosis and management of colorectal cancer: NICE clinical guideline 131 [online], (2011).
Brouquet, A. et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J. Am. Col. Surg. 210, 934–941 (2010).
Adam, R., Lucidi, V. & Bismuth, H. Hepatic colorectal metastases: methods of improving resectability. Surg. Clin. North Am. 84, 659–671 (2004).
de Haas, R. J. et al. Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal liver metastases. Br. J. Surg. 97, 1279–1289 (2010).
Hillingsø, J. G. & Wille-Jørgensen, P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer—a systematic review. Colorectal Dis. 11, 3–10 (2008).
Jegatheeswaran, S., Mason, J. M., Hancock, H. & Siriwardena, A. K. The liver-first approach to the management of colorectal cancer with synchronous hepatic metastases. JAMA Surg. 148, 385–391 (2013).
Mentha, G. et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before the treatment of colorectal primary. Br. J. Surg. 93, 872–878 (2006).
Akilu, M. & Eng, C. The current landscape of locally advanced rectal cancer. Nat. Rev. Clin. Oncol. 8, 649–659 (2011).
Sagar, J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Systemic Reviews, Issue 11. Art. No.: CD007378 http://dx.doi.10.1002/14651858.CD007378.pub2 (2011).
de Jong, M. C. et al. The liver-first approach for synchronous colorectal liver metastasis: a 5-year single-centre experience. HPB (Oxford) 13, 745–752 (2011).
O'Neill, B. D., Brown, G., Heald, R. J., Cunningham, D. & Tait, D. M. Non-operative treatment after neoadjuvant chemotherapy for rectal cancer. Lancet Oncol. 7, 625–633 (2007).
Slesser, A. A. et al. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg. Oncol. 22, 36–47 (2013).
Swan, P. J., Welsh, F. K., Chandrakumaran, K. & Rees, M. Long-term survival following delayed presentation and resection of colorectal liver metastases. Br. J. Surg. 98, 1309–1317 (2011).
Edge, S. B. et al. AJCC cancer staging manual 7th edn (Springer, 2010).
Mekenkamp, L. J. et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs. metachronous metastases. Br. J. Cancer 103, 159–164 (2010).
Sethi, N. & Kang, Y. Unravelling the complexity of metastasis—molecular understanding and targeted therapies. Nat. Rev. Cancer 11, 735–748 (2011).
van der Wal, G. et al. Angiogenesis in synchronous and metachronous colorectal liver metastases. The liver as permissive soil. Ann. Surg. 255, 86–94 (2012).
Wikman, H., Vessella, R. & Pantel, K. Cancer micrometastasis and tumour dormancy. APMIS 116, 754–770 (2008).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Hammoud, M. A., McCutcheon, I. E., Elsouki, R., Schoppa, D. & Patt, Y. Z. Colorectal carcinoma and brain metastases: distribution, treatment and survival. Ann. Surg. Oncol. 3, 453–463 (1996).
Karoui, M. et al. Stents for palliation of obstructive metastatic colon cancer. Arch. Surg. 142, 619–623 (2007).
Zhang, S., Gao, F., Luo, J. & Yang, J. Prognostic factors in survival of colorectal cancer patients with synchronous liver metastasis. Colorectal Dis. 12, 754–761 (2010).
Cirocchi, R. et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg. Oncol. 22, 14–21 (2013).
Amri, R., Bordeianou, L. G., Sylla, P. & Berger, D. L. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg. 148, 747–754 (2013).
Steele, R. J. et al. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 58, 530–535 (2009).
Logan, F. A. et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 61, 1439–1446 (2012).
NICE, National Institute for Health and Clinical Excellence. NICE clinical guidelines 104: Metastatic malignant disease of unknown primary origin: Diagnosis and management of metastatic malignant disease of unknown primary origin [online], (2010).
Hemminki, K., Riihimäki, M., Sundquist, K. & Hemminki, A. Site-specific survival rates for cancer of unknown primary according to location of metastases. Int. J. Cancer 133, 182–189 (2013).
Macmillan Cancer Support. About cancer of unknown primary [online], (2011).
Collins, R. et al. Emergency Surgery: Standards for unscheduled care (Royal College of Surgeons of England, 2011).
Ghazi, S. et al. Clinicopathological analysis of colorectal cancer: a comparison between emergency and elective surgical cases. World J. Surg. Oncol. 11, 133–144 (2013).
Seah, D. W., Ibrahim, S. & Tay, K. H. Hartmann procedure: is it still relevant today? ANZ J. Surg. 75, 436–440 (2005).
Meyer, F. et al. Emergency operation in carcinomas of the left colon: value of Hartmann's procedure. Tech. Coloproctol. 8 (Suppl. 1), S226–S229 (2004).
Ansaloni, L. et al. Guidelines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J. Emerg. Surg. 5, 29–39 (2010).
Tan, C. J., Dasari, B. V. & Gardiner, K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br. J. Surg. 99, 469–476 (2012).
Hill, J. Stenting and colorectal cancer. Br. J. Surg. 95, 1195–1196 (2008).
Fowler, K. J., Linehan, D. C. & Menias, C. O. Colorectal liver metastases: state of the art imaging. Ann. Surg. Oncol. 20, 1185–1193 (2013).
National Comprehensive Cancer Network Oncologic Guidelines. Colon cancer [online], (2013).
Berger-Kulemann, V. et al. Gadoxetic acid-enhanced 3.0T MR imaging versus multidetector-row CT in the detection of colorectal metastases in fatty liver using intraoperative ultrasound and histopathology as a standard of reference. Eur. J. Surg. Oncol. 38, 670–676 (2012).
Kulemann, V. et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur. J. Radiol. 79, e1–e6 (2011).
Kong, G. et al. The use of 18F-FDG PET/CT in colorectal liver metastases-comparison with CT and liver MRI. Eur. J. Nucl. Med. Mol. Imaging 35, 1323–1329 (2008).
Scharitzer, M. et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur. Radiol. 23, 2187–2196 (2013).
Akay, S., Kocaoglu, M., Emer, O., Battal, B. & Arslan, N. Diagnostic accuracy of whole-body diffusion-weighted magnetic resonance imaging with 3.0T in detection of primary and metastatic neoplasms. J. Med. Imaging Radiat. Oncol. 57, 274–282 (2013).
Marckmann, P. et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 17, 2359–2362 (2006).
Royal College of Radiologists. Evidence-based indications for the use of PET-CT in the United Kingdom 2012 [online], (2012).
Pawlik, T. et al. Trends in nontherapeutic laparotomy rates in patients undergoing surgical therapy for hepatic colorectal metastases. Ann. Surg. Oncol. 16, 371–378 (2009).
Adams, R. B. et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 15, 91–103 (2013).
The Royal College of Radiologists. Ultrasound training recommendations for medical and surgical specialties 2nd edn [online], (2012).
Westwood, M. et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol. Assess. 17, 1–243 (2013).
Gozzetti, G., Mazziotti, A. & Jovine, E. Intraoperative ultrasound—diagnostic aspects in liver surgery in Surgery of the Liver and Biliary Tract 2nd edn (ed. Blumgart, L. H.) 451–458 (Churchill Livingstone, 1994).
Mazzoni, G. et al. Intra-operative ultrasound for detection of liver metastases from colorectal cancer. Liver Int. 1, 88–94 (2008).
Garden, O. J. et al. Guidelines for resection of colorectal cancer liver metastases. Gut 55 (Suppl. 3), iii1–iii8 (2006).
Sorbye, H. et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann. Surg. 255, 534–539 (2012).
Garcea, G. et al. Improving the diagnostic yield from staging laparoscopy for periampullary malignancies: the value of preoperative inflammatory markers and radiological tumor size. Pancreas 41, 233–237 (2012).
Coburn, N. et al. Optimal management of gastric cancer: results from an international RAND/UCLA expert panel. Ann. Surg. 259, 102–108 (2014).
Dunne, D. F. et al. Routine staging laparoscopy has no place in the management of colorectal liver metastases. Eur. J. Surg. Oncol. 39, 721–725 (2013).
Bipat, S. et al. Evidence-base guideline on management of colorectal liver metastases in the Netherlands. Neth. J. Med. 65, 5–14 (2007).
Czoski-Murray, C. et al. What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature? Health Technol. Assess. 50, 1–159 (2012).
Pannell, L. M., Reyes, E. M. & Underwood, S. R. Cardiac risk assessment before non-cardiac surgery. Eur. Heart J. Cardiovasc. Imaging 4, 316–322 (2013).
Asli, I. et al. The utility and prognostic value of dipyridamole technetium-99m sestamibi myocardial perfusion imaging SPECT in predicting perioperative cardiac events following non-cardiac surgery. Perfusion 4, 333–339 (2013).
Junejo, M. et al. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br. J. Surg. 99, 1097–1104 (2012).
Vauthey, J. N. et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. 13, 2065–2072 (2006).
Zorzi, D. et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br. J. Surg. 3, 274–286 (2007).
Schwarz, R. E., Abdalla, E. K., Aloia, T. A. & Vauthey, J. N. AHPBA/SSO/SSAT sponsored consensus conference on the multidisciplinary treatment of colorectal cancer metastases. HPB (Oxford) 2, 89–90 (2013).
Belghiti, J. et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 2, 333–339 (2000).
Sigurdsson, H. K. et al. Palliative surgery for rectal cancer in a national cohort. Colorectal Dis. 4, 336–343 (2008).
Kobayashi, H. et al. Clinical benefit of surgery for stage IV colorectal cancer with synchronous peritoneal metastases. J. Gastroenterol. 49, 646–654 (2014).
Elias, D. M. & Ouellet, J. F. Incidence, distribution and significance of hilar lymph node metastases in hepatic colorectal metastases. Surg. Oncol. Clin. N. Am. 1, 221–229 (2003).
Treasure, T. & Utley, M. Surgical removal of asymptomatic pulmonary metastases: time for better evidence. BMJ 346, 824 (2013).
Limmer, S. et al. Sequential surgical resection of hepatic and pulmonary metastases from colorectal cancer. Langenbecks Arch. Surg. 395, 1128–1138 (2010).
Embún, R. et al. Pulmonary metastasectomy in colorectal cancer: a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR). BMJ Open 3, 1–12 (2013).
Jegatheeswaran, S., Satyadas, T., Sheen, A. J., Treasure, T. & Siriwardena, A. K. Thoracic surgical management of colorectal lung metastases: a questionnaire survey of members of the Society for Cardiothoracic Surgery in Great Britain and Ireland. Ann. R. Coll. Surg. Engl. 95, 140–143 (2013).
Treasure, T., Fallowfield, L., Lees, B. & Farewell, V. Pulmonary metastasectomy in colorectal cancer. Thorax 67, 185–187 (2012).
Pathak, S., Sarno, G., Nunes, Q. & Poston, G. J. Synchronous resection for colorectal liver metastases: the future. Eur. J. Surg. Oncol. 11, 1044–1046 (2010).
Spampinato, M. G., Mandalá, L., Quarta, G., Del Medico, P. & Baldazzi, G. One-stage, totally laparoscopic major hepatectomy and colectomy for colorectal neoplasm with synchronous liver metastasis: safety, feasibility and outcome. Surgery 6, 861–865 (2013).
Nordlinger, B. et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371, 1007–1016 (2008).
Nordlinger, B. et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 14, 1208–1215 (2013).
Chua, T. C., Saxena, A., Liauw, W., Kokandi, A. & Morris, D. L. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann. Surg. Oncol. 2, 492–501 (2010).
Primrose, J. N. et al. A randomized clinical trial of chemotherapy compared to chemotherapy in combination with cetuximab in k-RAS wild-type patients with operable metastases from colorectal cancer [abstract]. J. Clin. Oncol. 31 (Suppl.), a3504 (2013).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Normanno, N. et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat. Rev. Clin. Oncol. 9, 519–527 (2009).
Adam, R. et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann. Surg. 4, 644–657 (2004).
Petrelli, F. et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3,500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin. Colorectal Cancer 12, 145–151 (2013).
Kishi, Y. et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann. Surg. Oncol. 11, 2870–2876 (2010).
Folprecht, G. et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 11, 38–47 (2010).
Bischof, D. A., Clary, B. M., Maithel, S. K. & Pawlik, T. M. Surgical management of disappearing colorectal liver metastases. Br. J. Surg. 100, 1414–1420 (2013).
Hamady, Z. Z. et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann. Surg. 259, 543–548 (2014).
Sarpel, U. et al. Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann. Surg. Oncol. 2, 379–384 (2009).
Kulik, U., Bektas, H., Klempnauer, J. & Lehner, E. Repeat liver resection for colorectal metastases. Br. J. Surg. 7, 926–932 (2013).
Morris-Stiff, G., Gomez, D. & Prasad, R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J. Gastrointest. Surg. 2, 374–385 (2009).
Fung, J. et al. Use of liver stiffness measurement for liver resection surgery: correlation with indocyanine green clearance testing and post-operative outcome. PLoS ONE 28, e72306 (2013).
Wakiya, T. et al. Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-associated liver injury in patients with colorectal cancer liver metastasis. Ann. Surg. Oncol. 21, 167–172 (2014).
Ulla, M. et al. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterology 122, 337–342 (2013).
Broquet, A., Andreou, A., Shindo, J. & Vauthey, J. N. Methods to improve resectability in hepatocellular carcinoma. Recent Results Cancer Res. 190, 57–67 (2013).
Jamdar, S., Sheen, A. J. & Siriwardena, A. K. Colorectal liver metastases in Contemporary Coloproctology (eds Brown S. R. et al.) 201–213 (Springer-Verlag, 2012).
Shindoh, J. et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: towards zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J. Am. Coll. Surg. 2, 201–209 (2013).
Hoekstra, L. T. et al. Tumor progression after preoperative portal vein embolization. Ann. Surg. 5, 812–817 (2012).
Schnitzbauer, A. A. et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling two-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 255, 405–414 (2012).
Lam, V. W. et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 7, 483–491 (2013).
Karoui, M. et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br. J. Surg. 97, 1354–1362 (2010).
Abdalla, E. K. et al. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB (Oxford) 15, 119–130 (2013).
Decadt, B. & Siriwardena, A. K. Radiofrequency ablation of liver tumours: a systematic review. Lancet Oncol. 5, 550–560 (2004).
Lloyd, D. M. et al. International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 13, 579–585 (2011).
Aksoy, E. et al. Clinical scenarios associated with local recurrence after laparoscopic radiofrequency thermal ablation of colorectal liver metastases. Surgery 154, 748–752 (2013).
Scheele, J., Stangl, R., Altendorf-Hofmann, A. & Gall, F. P. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 110, 13–29 (1991).
Elias, D., Detroz, B., Lasser, P., Plaud, B. & Jerbi, G. Is simultaneous hepatectomy and intestinal anastomosis safe? Am. J. Surg. 169, 254–260 (1995).
Nordlinger, B. et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1,568 patients. Association Française de Chirurgie. Cancer 77, 1254–1262 (1996).
Martin, R. et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J. Am. Coll. Surg. 197, 233–242 (2003).
Weber, J. C., Bachellier, P., Oussoultzoglou, E. & Jaeck, D. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br. J. Surg. 90, 956–962 (2003).
Chua, H. K. et al. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis. Colon Rectum 47, 1310–1316 (2004).
Capussotti, L. et al. Major liver resections synchronous with colorectal surgery. Ann. Surg. Oncol. 14, 195–201 (2006).
Reddy, S. K. et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann. Surg. Oncol. 14, 3481–3491 (2007).
Vassiliou, I. et al. Surgical approaches of resectable synchronous colorectal liver metastases: timing considerations. World J. Gastroenterol. 13, 1431–1434 (2007).
Thelen, A. et al. Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer. Int. J. Colorectal Dis. 22, 1269–1276 (2007).
Turrini, O. et al. Strategies for the treatment of synchronous liver metastasis. Eur. J. Surg. Oncol. 33, 735–740 (2007).
Yan, T. D., Chu, F., Black, D., King, D. W. & Morris, D. L. Synchronous resection of colorectal primary cancer and liver metastases. World J. Surg. 31, 1496–1501 (2007).
Slupski, M., Wlodarczyk, Z., Jasinski, M., Masztalerz, M. & Tujakowsk, I. J. Outcomes of simultaneous and delayed resections of synchronous colorectal liver metastases. Can. J. Surg. 52, E241–E244 (2009).
Luo, Y. et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastases. J. Gastrointest. Surg. 14, 1974–1980 (2010).
Kaibori, M. et al. Timing of resection for synchronous liver metastases from colorectal cancer. Dig. Dis. Sci. 55, 3262–3270 (2010).
Broquet, A. et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J. Am. Coll. Surg. 210, 934–941 (2010).
Mentha, G. et al. 'Liver first' approach in the treatment of colorectal cancer with synchronous liver metastases. Dig. Surg. 25, 430–435 (2008).
Mentha, G. et al. Treatment strategies for the management of advanced colorectal liver metastases detected synchronously with the primary tumour. Eur. J. Surg. Oncol. 33, S76–S83 (2007).
Adam, R. et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 17, 1225–1239 (2012).
Author information
Authors and Affiliations
Contributions
A.K.S. and S.J. researched the data for the article, and A.K.S., J.M.M., S.M. and S.J. substantially contributed to discussion of content. A.K.S., J.M.M., S.M. and H.C.H. wrote the article, and all authors reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Siriwardena, A., Mason, J., Mullamitha, S. et al. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol 11, 446–459 (2014). https://doi.org/10.1038/nrclinonc.2014.90
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.90
This article is cited by
-
Impact of prognostic nutritional index change on prognosis after colorectal cancer surgery under propofol or sevoflurane anesthesia
BMC Anesthesiology (2024)
-
Prognostic potential of whole exome sequencing in the clinical management of metachronous colorectal cancer liver metastases
Cancer Cell International (2023)
-
Resection of the primary tumor improves the prognosis of gastrointestinal neuroendocrine neoplasms with liver metastases: mutual validation based on SEER database and institutional data
BMC Gastroenterology (2023)
-
A CT-based radiomics nomogram for predicting histopathologic growth patterns of colorectal liver metastases
Journal of Cancer Research and Clinical Oncology (2023)
-
The prognostic significance of clinicopathological characteristics in early-onset versus late-onset colorectal cancer liver metastases
International Journal of Colorectal Disease (2023)