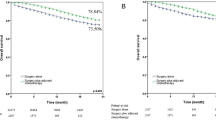
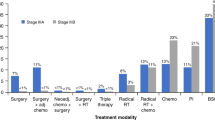
Abstract
The discovery in 2004 of activating mutations in the EGFR gene opened the era of personalized medicine in thoracic oncology. Treatment with drugs that target EGFR typically results in dramatic tumour response compared with conventional chemotherapy in patients with non-small-cell lung cancer. Subsequently, newer driver oncogenes such as ALK, ROS1 and RET have been discovered. Nevertheless, surgery has become safer and less invasive in the past 10–15 years. In the era of personalized medicine, thoracic surgeons have to think about their evolving roles. In this article, we discuss four topics relevant to this issue. Firstly, the value of surgical specimens as opposed to biopsy specimens for further understanding tumour biology is discussed. Secondly, extended indication of surgery in the era of targeted therapy is considered. Thirdly, in clinical trials that examine neoadjuvant therapy in patients selected by appropriate biomarkers, the important role of surgeons is highlighted. Finally, the possibility of personalizing the surgical procedure itself according to lung cancer subtypes defined by biomarkers is reviewed.
Key Points
-
Non-small-cell lung cancer (NSCLC)—especially adenocarcinoma—can be subdivided according to the presence of mutated driver oncogenes (for example, EGFR and ALK), which are crucial to efficient treatment selection
-
Surgery for NSCLC has become considerably safer and less invasive since the advent of optical, stapling and energy devices and progress in perioperative management
-
Surgical specimens are valuable for studying the genetics of this cancer type, which was shown to have more genetic alterations and to be more heterogeneous than previously thought
-
Surgery can be an efficient measure of treating patients with acquired resistance to targeted therapy
-
Surgery combined with drug therapy given either postoperatively or preoperatively for patients selected by distinct biomarkers is an attractive approach that is being tested
-
Personalization using biomarkers of surgical procedures remains an important subject for future research
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Travis, W. D. et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 6, 244–285 (2011).
Pao, W. & Girard, N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 12, 175–180 (2011).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Mitsudomi, T. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11, 121–128 (2010).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomized, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012).
Yang, J. C.-H. et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations [abstract]. J. Clin. Oncol. 30 (Suppl.), aLBA7500 (2012).
Schiller, J. H. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 346, 92–98 (2002).
Shaw, A. T. et al. Phase III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) (PROFILE 1007) [abstract]. Ann. Oncol. 23 (Suppl. 9), ixe21 (2012).
Sawabata, N. et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J. Thorac. Oncol. 6, 1229–1235 (2011).
Kuwano, H., Amano, J. & Yokomise, H. Thoracic and cardiovascular surgery in Japan during 2010: annual report by The Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 60, 680–708 (2012).
Yan, T. D., Black, D., Bannon, P. G. & McCaughan, B. C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J. Clin. Oncol. 27, 2553–2562 (2009).
Park, B. J., Zhang, H., Rusch, V. W. & Amar, D. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J. Thorac. Cardiovasc. Surg. 133, 775–779 (2007).
Martin-Ucar, A. E. et al. The beneficial effects of specialist thoracic surgery on the resection rate for non-small-cell lung cancer. Lung Cancer 46, 227–232 (2004).
Rivera, M. P. & Mehta, A. C. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132 (Suppl.), 131–148 (2007).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Tomiyama, N. et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur. J. Radiol. 59, 60–64 (2006).
Imielinski, M. et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 150, 1107–1120 (2012).
The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Kim, W. Y. & Kaelin, W. G. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 22, 4991–5004 (2004).
Schmid, K. et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin. Cancer Res. 15, 4554–4560 (2009).
Park, S. et al. Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. J. Thorac. Oncol. 4, 809–815 (2009).
Chang, Y. L., Wu, C. T., Shih, J. Y. & Lee, Y. C. Comparison of p53 and epidermal growth factor receptor gene status between primary tumors and lymph node metastases in non-small cell lung cancers. Ann. Surg. Oncol. 18, 543–550 (2011).
Chen, Z. Y. et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 17, 978–985 (2012).
Nakano, H. et al. Heterogeneity of epidermal growth factor receptor mutations within a mixed adenocarcinoma lung nodule. Lung Cancer 60, 136–140 (2008).
Sakurada, A., Lara-Guerra, H., Liu, N., Shepherd, F. A. & Tsao, M. S. Tissue heterogeneity of EGFR mutation in lung adenocarcinoma. J. Thorac. Oncol. 3, 527–529 (2008).
Taniguchi, K., Okami, J., Kodama, K., Higashiyama, M. & Kato, K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 99, 929–935 (2008).
Yatabe, Y., Matsuo, K. & Mitsudomi, T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J. Clin. Oncol. 29, 2972–2977 (2011).
Soh, J. et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells. PLoS ONE 4, e7464 (2009).
Yatabe, Y., Takahashi, T. & Mitsudomi, T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer Res. 68, 2106–2111 (2008).
Mitsudomi, T. & Yatabe, Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 98, 1817–1824 (2007).
Kosaka, T. et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin. Cancer Res. 12, 5764–5769 (2006).
Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 3, 75ra26 (2011).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Bivona, T. G. et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature 471, 523–526 (2011).
Ng, K. P. et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat. Med. 18, 521–528 (2012).
Faber, A. C. et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 1, 352–365 (2011).
Cheung, H. W. et al. Amplification of CRKL induces transformation and epidermal growth factor receptor inhibitor resistance in human non-small cell lung cancers. Cancer Discov. 1, 608–625 (2011).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Bean, J. et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl Acad. Sci. USA 104, 20932–20937 (2007).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007).
Suda, K., Mizuuchi, H., Maehara, Y. & Mitsudomi, T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation-diversity, ductility, and destiny. Cancer Metastasis Rev. 31, 807–814 (2012).
Suda, K. et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin. Cancer Res. 16, 5489–5498 (2010).
Scagliotti, G. V. et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 26, 3543–3551 (2008).
Yamamoto, N. et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 28, 3739–3745 (2010).
Ohe, Y. et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann. Oncol. 18, 317–323 (2007).
Peacock, C. D. & Watkins, D. N. Cancer stem cells and the ontogeny of lung cancer. J. Clin. Oncol. 26, 2883–2889 (2008).
Jones, R. J., Matsui, W. H. & Smith, B. D. Cancer stem cells: are we missing the target? J. Natl Cancer Inst. 96, 583–585 (2004).
Flanigan, R. C. et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J. Urol. 171, 1071–1076 (2004).
Yu, H. A. et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 8, 346–351 (2013).
Oxnard, G. R. et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin. Cancer Res. 17, 1616–1622 (2011).
Tomizawa, K. et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer 74, 139–144 (2011).
Takebe, N., Harris, P. J., Warren, R. Q. & Ivy, S. P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 8, 97–106 (2011).
Weichselbaum, R. R. & Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 8, 378–382 (2011).
Hellman, S. & Weichselbaum, R. R. Oligometastases. J. Clin. Oncol. 13, 8–10 (1995).
Pastorino, U. et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J. Thorac. Cardiovasc. Surg. 113, 37–49 (1997).
Hornbech, K., Ravn, J. & Steinbrüchel, D. A. Current status of pulmonary metastasectomy. Eur. J. Cardiothorac. Surg. 39, 955–962 (2011).
Yano, T. et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J. Surg. Oncol. 102, 852–855 (2010).
Lussier, Y. A. et al. MicroRNA expression characterizes oligometastasis(es). PLoS ONE 6, e28650 (2011).
Goldstraw, P. et al. Non-small-cell lung cancer. Lancet 378, 1727–1740 (2011).
Pignon, J. P. et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26, 3552–3559 (2008).
Postel-Vinay, S. et al. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat. Rev. Clin. Oncol. 9, 144–155 (2012).
Dematteo, R. P. et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373, 1097–1104 (2009).
Goss, G. D. et al. A phase III randomized, double-blind, placebo-controlled trial of the epidermal growth factor receptor inhibitor gefitinb in completely resected stage IB-IIIA non-small cell lung cancer (NSCLC): NCIC CTG BR.19 [abstract]. J. Clin. Oncol. 28 (Suppl. 18), LBA7005 (2010).
Kelly, K. et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. 26, 2450–2456 (2008).
D'Angelo, S. P. et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J. Thorac. Oncol. 7, 1815–1822 (2012).
Janjigian, Y. Y. et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J. Thorac. Oncol. 6, 569–575 (2011).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Tada, H. et al. Vinorelbine plus cisplatin versus gefitinib in resected non-small cell lung cancer haboring activating EGFR mutation (WJOG6410L) [abstract]. J. Clin. Oncol. 30 (Suppl.), TPS7110 (2012).
Altorki, N. et al. Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non-small-cell lung cancer. J. Clin. Oncol. 28, 3131–3137 (2010).
Takizawa, T. et al. Lymph node metastasis in small peripheral adenocarcinoma of the lung. J. Thorac. Cardiovasc. Surg. 116, 276–280 (1998).
Asamura, H. et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J. Thorac. Cardiovasc. Surg. 111, 1125–1134 (1996).
Ginsberg, R. J. & Rubinstein, L. V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 60, 615–622 (1995).
Suzuki, K. et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J. Thorac. Oncol. 6, 751–756 (2011).
Japan Clinical Oncology Group. JOCG [online], (2013).
Larsen, J. E. & Minna, J. D. Molecular biology of lung cancer: clinical implications. Clin. Chest Med. 32, 703–740 (2011).
Takeuchi, K. et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 18, 378–381 (2012).
Toyooka, S., Kiura, K. & Mitsudomi, T. EGFR mutation and response of lung cancer to gefitinib. N. Engl. J. Med. 352, 2136; author reply 2136 (2005).
Cappuzzo, F., Bemis, L. & Varella-Garcia, M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N. Engl. J. Med. 354, 2619–2621 (2006).
Acknowledgements
We thank Helena A. Yu and co-authors for sharing their manuscript before publication, and Masahiro Tsuboi and Pasi Janne for their helpful comments.
Author information
Authors and Affiliations
Contributions
T. Mitsudomi researched the data for the article, and all authors made a substantial contribution to discussion of the content. T. Mitsudomi and K. Suda wrote the article, and all authors reviewed and edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
T. Mitsudomi receives honoraria from AstraZeneca, Boehringer-Ingelheim, Chugai, Roche and Pfizer. His department receives research funding from AstraZeneca, Boehringer-Ingelheim, Chugai, and Pfizer. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mitsudomi, T., Suda, K. & Yatabe, Y. Surgery for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol 10, 235–244 (2013). https://doi.org/10.1038/nrclinonc.2013.22
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.22
This article is cited by
-
Neoadjuvant Afatinib for stage III EGFR-mutant non-small cell lung cancer: a phase II study
Nature Communications (2023)
-
Is there a role for lung surgery in initially unresectable non-small cell lung cancer after tyrosine kinase inhibitor treatment?
World Journal of Surgical Oncology (2022)
-
Postoperative Adverse Events are Associated with Oncologic Recurrence Following Curative-intent Resection for Lung Cancer
Lung (2020)
-
Prognostic value of radiomic analysis of iodine overlay maps from dual-energy computed tomography in patients with resectable lung cancer
European Radiology (2019)
-
Treatment of Advanced Non-Small Cell Lung Cancer in the Era of Targeted Therapy
Current Pulmonology Reports (2018)