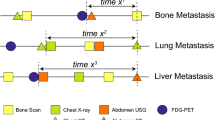
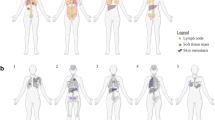
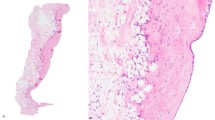
Key Points
-
Biopsies of suspected breast cancer metastases are not always performed in routine clinical practice owing to the cost and invasiveness of the procedure
-
Percutaneous image-guided biopsy is a safe, simple low-cost technique to obtain biopsy material from suspected metastases
-
Biopsies should be performed to determine oestrogen receptor, progesterone receptor or HER2 status of the lesion, particularly if the status of the primary tumour is unknown
-
Discordance between biomarker status in primary and secondary tumours can occur; however, standardized protocols might prevent discordance that results from technical errors
-
Increasing the numbers of biopsies performed throughout the disease trajectory will provide material for biobanks, aid future research, and might lead to innovative targeted therapies for breast cancer
Abstract
Biopsy of suspected metastases in patients with breast cancer is recommended in the practice guidelines of the National Comprehensive Cancer Network, but not always performed in routine oncology practice, often because of the cost and invasiveness of the procedure. Biopsies can confirm the presence of metastatic disease, reveal unsuspected benign disease or second (non-breast) malignancies and confirm expression of biomarkers, all of which can aid the optimal management of the cancer. Image-guided biopsy has increased the accuracy and safety of the procedure. Here, we aim to provide a practical algorithm for deciding when to perform biopsy of suspected breast cancer metastases, in order to optimize clinical practice. We expect that future clinical trials and standard-of-care practice will increasingly obtain tissue from metastases to assess molecular differences (DNA, RNA, protein) between the primary tumour and metastases. Advances in targeted therapy for breast cancer will be highly dependent on the availability of metastatic tissue. In this article, we provide an up-to-date review of the current issues in biopsy of suspected metastases in patients with breast cancer, including technical details of biopsy, pathology review of biopsy specimens, and interpretation of biopsy findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
National Comprehensive Cancer Network. Practice Guideline in Oncology: Breast Cancer v3 [online], (2012).
Cardoso, F., Harbeck, N., Fallowfield, L., Kyriakides, S. & Senkus, E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23 (Suppl. 7), vii11–vii19 (2012).
Pusztai, L., Viale, G., Kelly, C. M. & Hudis, C. A. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist 15, 1164–1168 (2010).
Wagner, H. N. Jr & Conti, P. S. Advances in medical imaging for cancer diagnosis and treatment. Cancer 67, 1121–1128 (1991).
Schnall, M. & Rosen, M. Primer on imaging technologies for cancer. J. Clin. Oncol. 24, 3225–3233 (2006).
Lavayssiere, R., Cabee, A. E. & Filmont, J. E. Positron emission tomography (PET) and breast cancer in clinical practice. Eur. J. Radiol. 69, 50–58 (2009).
Koh, D. M., Cook, G. J. & Husband, J. E. New horizons in oncologic imaging. N. Engl. J. Med. 348, 2487–2488 (2003).
Li, F., Engelmann, R., Doi, K. & MacMahon, H. Improved detection of small lung cancers with dual-energy subtraction chest radiography. AJR Am. J. Roentgenol. 190, 886–891 (2008).
Mayo-Smith, W. W., Boland, G. W., Noto, R. B. & Lee, M. J. State-of-the-art adrenal imaging. Radiographics 21, 995–1012 (2001).
Simmons, C. et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann. Oncol. 20, 1499–1504 (2009).
Gupta, S. & Madoff, D. C. Image-guided percutaneous needle biopsy in cancer diagnosis and staging. Tech. Vasc. Interv. Radiol. 10, 88–101 (2007).
Gupta, S. New techniques in image-guided percutaneous needle biopsy. Cardiovasc. Intervent. Radiol. 27, 91–104 (2004)
Silverman, S. G. et al. Percutaneous abdominal biopsy: cost-identification analysis. Radiology 206, 429–435 (1998).
Gupta, S. et al. Quality improvement guidelines for percutaneous needle biopsy. J. Vasc. Interv. Radiol. 21, 969–975 (2010).
Liberman, L. Centennial dissertation. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. AJR Am. J. Roentgenol. 174, 1191–1199 (2000).
Pasha, T., Gabriel, S., Therneau, T., Dickson, E. R. & Lindor, K. D. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 27, 1220–1226 (1998).
Gani, M. S., Shafee, A. M. & Soliman, I. Y. Ultrasound guided percutaneous fine needle aspiration biopsy/automated needle core biopsy of abdominal lesions: effect on management and cost effectiveness. Ann. Afr. Med. 10, 133–138 (2011).
Dodd, G. D. 3rd et al. Sonography: the undiscovered jewel of interventional radiology. Radiographics 16, 1271–1288 (1996).
Stattaus, J. et al. MR-guided liver biopsy within a short, wide-bore 1.5 Tesla MR system. Eur. Radiol. 18, 2865–2873 (2008).
Kariniemi, J., Blanco Sequeiros, R., Ojala, R. & Tervonen, O. MRI-guided abdominal biopsy in a 0.23-T open-configuration MRI system. Eur. Radiol. 15, 1256–1262 (2005).
Yakar, D. et al. Feasibility of 3T dynamic contrast-enhanced magnetic resonance-guided biopsy in localizing local recurrence of prostate cancer after external beam radiation therapy. Invest. Radiol. 45, 121–125 (2010).
DiBonito, L., Falconieri, G., Colautti, I., Bonifacio, D. & Dudine, S. The positive peritoneal effusion. A retrospective study of cytopathologic diagnoses with autopsy confirmation. Acta Cytol. 37, 483–488 (1993).
Runyon, B. A., Hoefs, J. C. & Morgan, T. R. Ascitic fluid analysis in malignancy-related ascites. Hepatology 8, 1104–1109 (1988).
Hilton, J. F. et al. Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Res. Treat. 129, 761–765 (2011).
Amir, E. et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat. Rev. 38, 708–714 (2012).
Bussolati, G. & Leonardo, E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J. Clin. Pathol. 61, 1184–1192 (2008).
Moulton, J. S. & Moore, P. T. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology 186, 515–522 (1993).
Yamagami, T., Iida, S., Kato, T., Tanaka, O. & Nishimura, T. combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? AJR Am. J. Roentgenol. 180, 811–815 (2003).
Tsang, P. et al. Image-directed percutaneous biopsy with large-core needles. Comparison of cytologic and histologic findings. Acta Cytol. 39, 752–758 (1995).
Yao, X. et al. Fine-needle aspiration biopsy versus core-needle biopsy in diagnosing lung cancer: a systematic review. Curr. Oncol. 19, e16–e27 (2012).
Mullink, H., Henzen-Logmans, S. C., Tadema, T. M., Mol, J. J. & Meijer, C. J. Influence of fixation and decalcification on the immunohistochemical staining of cell-specific markers in paraffin-embedded human bone biopsies. J. Histochem. Cytochem. 33, 1103–1109 (1985).
Aurilio, G. et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. http://dx.doi.org/10.3109/0284186X.2012.754990
Liebens, F. et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas 62, 113–123 (2009).
Amir, E. et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 30, 587–592 (2012).
Sauter, G., Lee, J., Bartlett, J. M. S., Slamon, D. J. & Press, M. F. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J. Clin. Oncol. 27, 1323–1333 (2009).
Hanley, K. Z., Birdsong, G. G., Cohen, C. & Siddiqui, M. T. Immunohistochemical detection of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast carcinomas: comparison on cell block, needle-core, and tissue block preparations. Cancer Cytopathol. 117, 279–288 (2009).
Perez, E. A. et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J. Clin. Oncol. 24, 3032–3038 (2006).
Kocjan, G. et al. BSCC Code of Practice--fine needle aspiration cytology. Cytopathology 20, 283–296 (2009).
Austin, J. H. & Cohen, M. B. Value of having a cytopathologist present during percutaneous fine-needle aspiration biopsy of lung: report of 55 cancer patients and metaanalysis of the literature. AJR Am. J. Roentgenol. 160, 175–177 (1993).
Charboneau, J. W., Reading, C. C. & Welch, T. J. CT and sonographically guided needle biopsy: current techniques and new innovations. AJR Am. J. Roentgenol. 154, 1–10 (1990).
Reading, C. C., Charboneau, J. W., James, E. M. & Hurt, M. R. Sonographically guided percutaneous biopsy of small (3 cm or less) masses. AJR Am. J. Roentgenol. 151, 189–192 (1988).
Beslic, S., Zukic, F. & Milisic, S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol. Oncol. 46, 19–22 (2012).
Hopper, K. D. et al. Blinded comparison of biopsy needles and automated devices in vitro: 2. Biopsy of medical renal disease. AJR Am. J. Roentgenol. 161, 1299–1301 (1993).
Hudock, J. A., Hanau, C. A., Christen, R. & Bibbo, M. Expression of estrogen and progesterone receptors in cytologic specimens using various fixatives. Diagn. Cytopathol. 15, 78–83 (1996).
Gong, Y., Symmans, W. F., Krishnamurthy, S., Patel, S. & Sneige, N. Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer 102, 34–40 (2004).
Hanley, K. Z., Siddiqui, M. T., Lawson, D., Cohen, C. & Nassar, A. Evaluation of new monoclonal antibodies in detection of estrogen receptor, progesterone receptor, and Her2 protein expression in breast carcinoma cell block sections using conventional microscopy and quantitative image analysis. Diagn. Cytopathol. 37, 251–257 (2009).
Beatty, B. G. et al. HER-2/neu detection in fine-needle aspirates of breast cancer: fluorescence in situ hybridization and immunocytochemical analysis. Am. J. Clin. Pathol. 122, 246–255 (2004).
Yaziji, H. et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 291, 1972–1977 (2004).
Goldstein, N. S., Ferkowicz, M., Odish, E., Mani, A. & Hastah, F. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am. J. Clin. Pathol. 120, 86–92 (2003).
Liedtke, C. et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann. Oncol. 20, 1953–1958 (2009).
Khoury, T. et al. Delay to formalin fixation effect on breast biomarkers. Mod. Pathol. 22, 1457–1467 (2009).
Amir, E. & Clemons, M. Should a biopsy be recommended to confirm metastatic disease in women with breast cancer? Lancet Oncol. 10, 933–935 (2009).
Lindstrom, L. S. et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 30, 2601–2608 (2012).
Thompson, A. M. et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 12, R92 (2010).
Heitz, F. et al. Differences in the receptor status between primary and recurrent breast cancer—the frequency of and the reasons for discordance. Oncology 84, 319–325 (2013).
Ibrahim, T. et al. Hormonal receptor, human epidermal growth factor receptor-2, and Ki67 discordance between primary breast cancer and paired metastases: clinical impact. Oncology 84, 150–157 (2013).
Masood, S. & Bui, M. M. Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann. Clin. Lab. Sci. 30, 259–265 (2000).
Aitken, S. J., Thomas, J. S., Langdon, S. P., Harrison, D. J. & Faratian, D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann. Oncol. 21, 1254–1261 (2010).
Amir, E. et al. Discordance between receptor status in primary and metastatic breast cancer: an exploratory study of bone and bone marrow biopsies. Clin. Oncol. (R. Coll. Radiol.) 20, 763–768 (2008).
Aoyama, K., Kamio, T., Nishikawa, T. & Kameoka, S. A comparison of HER2/neu gene amplification and its protein overexpression between primary breast cancer and metastatic lymph nodes. Jpn J. Clin. Oncol. 40, 613–619 (2010).
Azam, M., Qureshi, A. & Mansoor, S. Comparison of estrogen receptors, progesterone receptors and HER-2/neu expression between primary and metastatic breast carcinoma. J. Pak. Med. Assoc. 59, 736–740 (2009).
Wilking, U. et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res. Treat. 125, 553–561 (2010).
Wu, J. M. et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin. Cancer Res. 14, 1938–1946 (2008).
Zidan, J. et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 93, 552–556 (2005).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Simon, R. et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J. Natl Cancer Inst. 93, 1141–1146 (2001).
Gancberg, D. et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann. Oncol. 13, 1036–1043 (2002).
Tanner, M., Jarvinen, P. & Isola, J. Amplification of HER-2/neu and topoisomerase IIα in primary and metastatic breast cancer. Cancer Res. 61, 5345–5348 (2001).
Taucher, S. et al. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER2/neu in patients with breast cancer. Breast Cancer Res. Treat. 82, 207–213 (2003).
Xu, R. et al. Amplification of Her-2/neu gene in Her-2/neu-overexpressing and -nonexpressing breast carcinomas and their synchronous benign, premalignant, and metastatic lesions detected by FISH in archival material. Mod. Pathol. 15, 116–124 (2002).
Carlsson, J. et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br. J. Cancer 90, 2344–2348 (2004).
Regitnig, P., Schippinger, W., Lindbauer, M., Samonigg, H. & Lax, S. F. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J. Pathol. 203, 918–926 (2004).
Gong, Y., Booser, D. J. & Sneige, N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer 103, 1763–1769 (2005).
Pectasides, D. et al. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res. 26, 647–653 (2006).
D'Andrea, M. R. et al. Correlation between genetic and biological aspects in primary non-metastatic breast cancers and corresponding synchronous axillary lymph node metastasis. Breast Cancer Res. Treat. 101, 279–284 (2007).
Lower, E. E., Glass, E., Blau, R. & Harman, S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res. Treat. 113, 301–306 (2008).
Lower, E. E., Glass, E. L., Bradley, D. A., Blau, R. & Heffelfinger, S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res. Treat. 90, 65–70 (2005).
Barry, W. T. et al. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J. Clin. Oncol. 28, 2198–2206 (2010).
Johnston, S. R. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin. Cancer Res. 12, 1061s–1068s (2006).
Khasraw, M., Brogi, E. & Seidman, A. D. The need to examine metastatic tissue at the time of progression of breast cancer: is re-biopsy a necessity or a luxury? Curr. Oncol. Rep. 13, 17–25 (2011).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 366, 520–529 (2012).
Hammond, M. E. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795 (2010).
Iwamoto, T. et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 30, 729–734 (2012).
Kuukasjarvi, T. et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 57, 1597–1604 (1997).
Chang, H. J. et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J. Clin. Oncol. 41, 593–599 (2011).
Gong, Y., Han, E. Y., Guo, M., Pusztai, L. & Sneige, N. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer 117, 705–713 (2011).
Idirisinghe, P. K. et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am. J. Clin. Pathol. 133, 416–429 (2010).
Sauter, G., Lee, J., Bartlett, J. M., Slamon, D. J. & Press, M. F. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J. Clin. Oncol. 27, 1323–1333 (2009).
Shimizu, C. et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J. Surg. Oncol. 73, 17–20 (2000).
Thompson, A. M. et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 12, R92 (2010).
Tsutsui, S., Ohno, S., Murakami, S., Hachitanda, Y. & Oda, S. Prognostic value of c-erbB2 expression in breast cancer. J. Surg. Oncol. 79, 216–223 (2002).
Burstein, H. J. et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J. Clin. Oncol. 21, 46–53 (2003).
Harris, L. N. et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin. Cancer Res. 13, 1198–1207 (2007).
Hurley, J. et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J. Clin. Oncol. 24, 1831–1838 (2006).
Mittendorf, E. A. et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 15, 7381–7388 (2009).
Niikura, N. et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 30, 593–599 (2012).
Houssami, N., Macaskill, P., Balleine, R. L., Bilous, M. & Pegram, M. D. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res. Treat. 129, 659–674 (2011).
Kuukasjarvi, T., Kononen, J., Helin, H., Holli, K. & Isola, J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 14, 2584–2589 (1996).
Klein, C. A. et al. Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc. Natl Acad. Sci. USA 96, 4494–4499 (1999).
Gelao, L. et al. Tumour dormancy and clinical implications in breast cancer. Ecancermedicalscience 7, 320 (2013).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353–360 (2012).
Banerji, S. et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486, 405–409 (2012).
Meric-Bernstam, F., Farhangfar, C., Mendelsohn, J. & Mills, G. B. Building a personalized medicine infrastructure at a major cancer center. J. Clin. Oncol. 31, 1849–1857 (2013).
Oliveira, M. C., Neto, C., Ribeiro Morais, G. & Thiemann, T. Steroid receptor ligands for breast cancer targeting: an insight into their potential role as PET imaging agents. Curr. Med. Chem. 20, 222–245 (2013).
Kelloff, G. J. et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin. Cancer Res. 11, 7967–7985 (2005).
De Mattos-Arruda, L. et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat. Rev. Clin. Oncol. 10, 377–389 (2013).
Acknowledgements
N. Niikura was supported in part by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities. We thank S. Deming, Scientific Publications, MD Anderson Cancer Center, for editorial assistance.
Author information
Authors and Affiliations
Contributions
N. Niikura, B. C. Odisio, F. W. Symmans and N. T. Ueno researched data for the article. All authors wrote the article, made substantial contribution to discussion of the content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N. T. Ueno receives grant funding from ApoCell Inc. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Niikura, N., Odisio, B., Tokuda, Y. et al. Latest biopsy approach for suspected metastases in patients with breast cancer. Nat Rev Clin Oncol 10, 711–719 (2013). https://doi.org/10.1038/nrclinonc.2013.182
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.182
This article is cited by
-
Diagnostic performance of 18F-fluorodeoxyglucose PET/CT and bone scintigraphy in breast cancer patients with suspected bone metastasis
Breast Cancer (2016)
-
Using tumour phylogenetics to identify the roots of metastasis in humans
Nature Reviews Clinical Oncology (2015)