Abstract
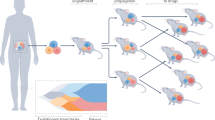
Progress in oncology drug development has been hampered by a lack of preclinical models that reliably predict clinical activity of novel compounds in cancer patients. In an effort to address these shortcomings, there has been a recent increase in the use of patient-derived tumour xenografts (PDTX) engrafted into immune-compromised rodents such as athymic nude or NOD/SCID mice for preclinical modelling. Numerous tumour-specific PDTX models have been established and, importantly, they are biologically stable when passaged in mice in terms of global gene-expression patterns, mutational status, metastatic potential, drug responsiveness and tumour architecture. These characteristics might provide significant improvements over standard cell-line xenograft models. This Review will discuss specific PDTX disease examples illustrating an overview of the opportunities and limitations of these models in cancer drug development, and describe concepts regarding predictive biomarker development and future applications.
Key Points
-
Many preclinical animal models fail to accurately predict the clinical efficacy of novel anticancer agents, largely due to their inability to reflect the complexity and heterogeneity of human tumours
-
Patient-derived tumour xenograft models (PDTX), where surgically resected tumour samples are engrafted directly into immune-compromised mice, offer several advantages over standard cell-line xenograft models
-
PDTX tumours maintain the molecular, genetic and histological heterogeneity typical of tumours of origin through serial passaging in mice
-
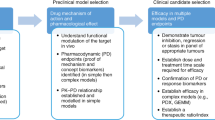
The tumour histology of PDTX models provides an excellent in vivo preclinical platform to study cancer stem-cell biology and stromal–tumour interactions; novel cancer therapeutics can also be assessed
-
Well-characterized PDTX models represent an information-rich preclinical resource for analysis of drug activity, including novel–novel drug combinations, as well as predictive biomarker discovery
-
The PDTX approach to modelling of specific cancer types could potentially reduce non-informative animal studies while providing a more-relevant system to test clinically directed hypotheses
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Johnson, J. I. et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer 84, 1424–1431 (2001).
Daniel, V. C. et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 69, 3364–3373 (2009).
Giovanella, B. C. et al. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science 246, 1046–1048 (1989).
Houghton, J. A., Maroda, S. J. Jr, Phillips, J. O. & Houghton, P. J. Biochemical determinants of responsiveness to 5-fluorouracil and its derivatives in xenografts of human colorectal adenocarcinomas in mice. Cancer Res. 41, 144–149 (1981).
Houghton, J. A. & Taylor, D. M. Growth characteristics of human colorectal tumours during serial passage in immune-deprived mice. Br. J. Cancer 37, 213–223 (1978).
Jin, K. et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin. Transl. Oncol. 12, 473–480 (2010).
Morton, C. L. & Houghton, P. J. Establishment of human tumor xenografts in immunodeficient mice. Nat. Protoc. 2, 247–250 (2007).
Rubio-Viqueira, B. & Hidalgo, M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin. Pharmacol. Ther. 85, 217–221 (2009).
Sausville, E. A. & Burger, A. M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 66, 3351–3354 (2006).
Jin, K. et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin. Transl. Oncol. 12, 473–480 (2010).
Rubio-Viqueira, B. et al. An in vivo platform for translational drug development in pancreatic cancer. Clin. Cancer Res. 12, 4652–4661 (2006).
John, T. et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin. Cancer Res. 17, 134–141 (2011).
Merk, J., Rolff, J., Becker, M., Leschber, G. & Fichtner, I. Patient-derived xenografts of non-small-cell lung cancer: a pre-clinical model to evaluate adjuvant chemotherapy? Eur. J. Cardiothorac. Surg. 36, 454–459 (2009).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Simpson-Abelson, M. R. et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rγnull mice. J. Immunol. 180, 7009–7018 (2008).
Pitts, T. M. et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: approach to individualized therapy in early development. Clin. Cancer Res. 16, 3193–3204 (2010).
Sanz, L. et al. Differential transplantability of human endothelial cells in colorectal cancer and renal cell carcinoma primary xenografts. Lab. Invest. 89, 91–97 (2009).
Gray, D. R. et al. Short-term human prostate primary xenografts: an in vivo model of human prostate cancer vasculature and angiogenesis. Cancer Res. 64, 1712–1721 (2004).
Smith, V., Wirth, G. J., Fiebig, H. H. & Burger, A. M. Tissue microarrays of human tumor xenografts: characterization of proteins involved in migration and angiogenesis for applications in the development of targeted anticancer agents. Cancer Genomics Proteomics 5, 263–273 (2008).
Garrido-Laguna, I. et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin. Cancer Res. 17, 5793–5800 (2011).
Fichtner, I. et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 14, 6456–6468 (2008).
Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008).
Linnebacher, M. et al. Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer 10, 362 (2010).
Dangles-Marie, V. et al. Establishment of human colon cancer cell lines from fresh tumors versus xenografts: comparison of success rate and cell line features. Cancer Res. 67, 398–407 (2007).
Guenot, D. et al. Primary tumour genetic alterations and intra-tumoral heterogeneity are maintained in xenografts of human colon cancers showing chromosome instability. J. Pathol. 208, 643–652 (2006).
Fichtner, I. et al. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur. J. Cancer 40, 298–307 (2004).
Krumbach, R. et al. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur. J. Cancer 47, 1231–1243 (2011).
Bertotti, A. et al. A molecularly annotated platform of patient-derived xenografts ('xenopatients') identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 1, 508–523 (2011).
Tentler, J. J. et al. Identification of predictive markers of response to the MEK1/2 inhibitor selumetinib (AZD6244) in K-ras-mutated colorectal cancer. Mol. Cancer Ther. 9, 3351–3362 (2010).
Arcaroli, J. J. et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin. Cancer Res. 16, 4165–4177 (2010).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA 104, 10158–10163 (2007).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Kim, M. P. et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 4, 1670–1680 (2009).
Fu, X., Guadagni, F. & Hoffman, R. M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl Acad. Sci. USA 89, 5645–5649 (1992).
Garrido-Laguna, I. et al. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br. J. Cancer 103, 649–655 (2010).
Rubio-Viqueira, B. et al. Optimizing the development of targeted agents in pancreatic cancer: tumor fine-needle aspiration biopsy as a platform for novel prospective ex vivo drug sensitivity assays. Mol. Cancer Ther. 6, 515–523 (2007).
Jimeno, A. et al. A fine-needle aspirate-based vulnerability assay identifies polo-like kinase 1 as a mediator of gemcitabine resistance in pancreatic cancer. Mol. Cancer Ther. 9, 311–318 (2010).
Hidalgo, M. et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol. Cancer Ther. 10, 1311–1316 (2011).
Villarroel, M. C. et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol. Cancer Ther. 10, 3–8 (2011).
Jones, S. et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324, 217 (2009).
Von Hoff, D. D. et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic Cancer: a phase I/II trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Bailey, J. M. et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 14, 5995–6004 (2008).
Yamazaki, S. et al. Pharmacokinetic-pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and anti-tumor efficacy in human tumor xenograft mouse models. J. Pharmacol. Exp. Ther. 340, 549–557 (2012).
Christensen, J. G. Proof of principle for crizotinib in anaplastic lymphoma kinase-positive malignancies was achieved in ALK-positive nonclinical models. Mol. Cancer Ther. 10, 2024 (2011).
Sasaki, T. et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 71, 6051–6060 (2011).
Ercan, D. et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene 29, 2346–2356 (2010).
Yoshida, T. et al. Effects of Src inhibitors on cell growth and epidermal growth factor receptor and MET signaling in gefitinib-resistant non-small cell lung cancer cells with acquired MET amplification. Cancer Sci. 101, 167–172 (2010).
Dong, X. et al. Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 16, 1442–1451 (2010).
Nemati, F. et al. Preclinical assessment of cisplatin-based therapy versus docetaxel-based therapy on a panel of human non-small-cell lung cancer xenografts. Anticancer Drugs 20, 932–940 (2009).
Cutz, J. C. et al. Establishment in severe combined immunodeficiency mice of subrenal capsule xenografts and transplantable tumor lines from a variety of primary human lung cancers: potential models for studying tumor progression-related changes. Clin. Cancer Res. 12, 4043–4054 (2006).
Taetle, R. et al. Use of nude mouse xenografts as preclinical screens. Characterization of xenograft-derived melanoma cell lines. Cancer 60, 1836–1841 (1987).
Fiebig, H. H. et al. Gene signatures developed from patient tumor explants grown in nude mice to predict tumor response to 11 cytotoxic drugs. Cancer Genomics Proteomics 4, 197–209 (2007).
Schatton, T. et al. Identification of cells initiating human melanomas. Nature 451, 345–349 (2008).
Nemati, F. et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin. Cancer Res. 16, 2352–2362 (2010).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Hennessey, P. T. et al. Promoter methylation in head and neck squamous cell carcinoma cell lines is significantly different than methylation in primary tumors and xenografts. PLoS ONE 6, e20584 (2011).
Prince, M. E. et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl Acad. Sci. USA 104, 973–978 (2007).
Chen, J., Milo, G. E., Shuler, C. F. & Schuller, D. E. Xenograft growth and histodifferentiation of squamous cell carcinomas of the pharynx and larynx. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 81, 197–202 (1996).
Zatterstrom, U. K. et al. Growth of xenografted squamous cell carcinoma of the head and neck--possible correlation with patient survival. APMIS 100, 976–980 (1992).
Wennerberg, J., Trope, C. & Biorklund, A. Heterotransplantation of human head and neck tumours into nude mice. Acta Otolaryngol. 95, 183–190 (1983).
Langdon, S. P. et al. Preclinical phase II studies in human tumor xenografts: a European multicenter follow-up study. Ann. Oncol. 5, 415–422 (1994).
Henriksson, E. et al. p53 mutation and cyclin D1 amplification correlate with cisplatin sensitivity in xenografted human squamous cell carcinomas from head and neck. Acta Oncol. 45, 300–305 (2006).
Peltonen, J. K. et al. Specific TP53 mutations predict aggressive phenotype in head and neck squamous cell carcinoma: a retrospective archival study. Head Neck Oncol. 3, 20 (2011).
Cabelguenne, A. et al. p53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: a prospective series. J. Clin. Oncol. 18, 1465–1473 (2000).
Koch, W. M. et al. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J. Natl Cancer Inst. 88, 1580–1586 (1996).
US National Library of Medicine. ClinicalTrials.gov[online], (2011).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
Carey, L. A. et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295, 2492–2502 (2006).
Beckhove, P. et al. Efficient engraftment of human primary breast cancer transplants in nonconditioned NOD/Scid mice. Int. J. Cancer 105, 444–453 (2003).
de Plater, L. et al. Establishment and characterisation of a new breast cancer xenograft obtained from a woman carrying a germline BRCA2 mutation. Br. J. Cancer 103, 1192–1200 (2010).
DeRose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 17, 1514–1520 (2011).
Marangoni, E. et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 13, 3989–3998 (2007).
Moestue, S. A. et al. Distinct choline metabolic profiles are associated with differences in gene expression for basal-like and luminal-like breast cancer xenograft models. BMC Cancer 10, 433 (2010).
Laitinen, S., Karhu, R., Sawyers, C. L., Vessella, R. L. & Visakorpi, T. Chromosomal aberrations in prostate cancer xenografts detected by comparative genomic hybridization. Genes Chromosomes Cancer 35, 66–73 (2002).
Gray, D. R. et al. Short-term human prostate primary xenografts: an in vivo model of human prostate cancer vasculature and angiogenesis. Cancer Res. 64, 1712–1721 (2004).
Grisanzio, C. et al. Orthotopic xenografts of RCC retain histological, immunophenotypic and genetic features of tumours in patients. J. Pathol. 225, 212–221 (2011).
Yoshida, T. et al. Antiandrogen bicalutamide promotes tumor growth in a novel androgen-dependent prostate cancer xenograft model derived from a bicalutamide-treated patient. Cancer Res. 65, 9611–9616 (2005).
Wang, Y. et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 64, 149–159 (2005).
Coppin, C., Kollmannsberger, C., Le, L., Porzsolt, F. & Wilt, T. J. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 108, 1556–1563 (2011).
Beniers, A. J. et al. Establishment and characterization of five new human renal tumor xenografts. Am. J. Pathol. 140, 483–495 (1992).
Kopper, L. et al. Renal cell carcinoma--xenotransplantation into immuno-suppressed mice. Oncology 41, 19–24 (1984).
Beroukhim, R. et al. Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 69, 4674–4681 (2009).
An, Z., Jiang, P., Wang, X., Moossa, A. R. & Hoffman, R. M. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin. Exp. Metastasis 17, 265–270 (1999).
Angevin, E. et al. Human renal cell carcinoma xenografts in SCID mice: tumorigenicity correlates with a poor clinical prognosis. Lab. Invest. 79, 879–888 (1999).
Yuen, J. S. et al. Inhibition of angiogenic and non-angiogenic targets by sorafenib in renal cell carcinoma (RCC) in a RCC xenograft model. Br. J. Cancer 104, 941–947 (2011).
Hammers, H. J. et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol. Cancer Ther. 9, 1525–1535 (2010).
Ellis, L. et al. Vascular disruption in combination with mTOR inhibition in renal cell carcinoma. Mol. Cancer Ther. 11, 383–392 (2012).
Keunen, O. et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl Acad. Sci. USA 108, 3749–3754 (2011).
Wang, J. et al. A reproducible brain tumour model established from human glioblastoma biopsies. BMC Cancer 9, 465 (2009).
Carol, H. et al. Initial testing of topotecan by the pediatric preclinical testing program. Pediatr. Blood Cancer 54, 707–715 (2010).
Houghton, P. J. et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother. Pharmacol. 36, 393–403 (1995).
Vassal, G. et al. Potent therapeutic activity of irinotecan (CPT-11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int. J. Cancer 73, 156–163 (1997).
Houghton, P. J. et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr. Blood Cancer 49, 928–940 (2007).
Foreman, N. K., Love, S. & Thorne, R. Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatr. Neurosurg. 24, 119–125 (1996).
Merchant, T. E. et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J. Clin. Oncol. 22, 3156–3162 (2004).
Pollack, I. F. et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery 37, 655–666 (1995).
Yu, L. et al. A clinically relevant orthotopic xenograft model of ependymoma that maintains the genomic signature of the primary tumor and preserves cancer stem cells in vivo. Neuro. Oncol. 12, 580–594 (2010).
Zembutsu, H. et al. Genome-wide cDNA microarray screening to correlate gene expression profiles with sensitivity of 85 human cancer xenografts to anticancer drugs. Cancer Res. 62, 518–527 (2002).
Tan, A. C., Naiman, D. Q., Xu, L., Winslow, R. L. & Geman, D. Simple decision rules for classifying human cancers from gene expression profiles. Bioinformatics 21, 3896–3904 (2005).
Jimeno, A. et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res. 68, 2841–2849 (2008).
Messersmith, W. A. et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol. Cancer Ther. 8, 1484–1493 (2009).
Rajeshkumar, N. V. et al. Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin. Cancer Res. 15, 4138–4146 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2010).
US National Library of Medicine. ClinicalTrials.gov[online], (2010).
Singh, M. et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat. Biotechnol. 28, 585–593 (2010).
Giovanella, B. C., Stehlin, J. S., Jr, Shepard, R. C. & Williams, L. J. Jr. Correlation between response to chemotherapy of human tumors in patients and in nude mice. Cancer 52, 1146–1152 (1983).
Tentler, J. J. et al. Assessment of the in vivo antitumor effects of ENMD-2076, a novel multitargeted kinase inhibitor, against primary and cell line-derived human colorectal cancer xenograft models. Clin. Cancer Res. 16, 2989–2998 (2010).
Rajeshkumar, N. V. et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin. Cancer Res. 17, 2799–2806 (2011).
Song, D. et al. Antitumor activity and molecular effects of the novel heat shock protein 90 inhibitor, IPI-504, in pancreatic cancer. Mol. Cancer Ther. 7, 3275–3284 (2008).
Merk, J., Rolff, J., Dorn, C., Leschber, G. & Fichtner, I. Chemoresistance in non-small-cell lung cancer: can multidrug resistance markers predict the response of xenograft lung cancer models to chemotherapy? Eur. J. Cardiothorac. Surg. 40, e29–e33 (2011).
Hammer, S. et al. Comparative profiling of the novel epothilone, sagopilone, in xenografts derived from primary non-small cell lung cancer. Clin. Cancer Res. 16, 1452–1465 (2010).
Kolfschoten, G. M. et al. Development of a panel of 15 human ovarian cancer xenografts for drug screening and determination of the role of the glutathione detoxification system. Gynecol. Oncol. 76, 362–368 (2000).
Huynh, H., Soo, K. C., Chow, P. K., Panasci, L. & Tran, E. Xenografts of human hepatocellular carcinoma: a useful model for testing drugs. Clin. Cancer Res. 12, 4306–4314 (2006).
Huynh, H. et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin. Cancer Res. 14, 6146–6153 (2008).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article, including researching data, discussion of content, writing, reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tentler, J., Tan, A., Weekes, C. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 9, 338–350 (2012). https://doi.org/10.1038/nrclinonc.2012.61
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.61
This article is cited by
-
Anti-cancer effect of afatinib, dual inhibitor of HER2 and EGFR, on novel mutation HER2 E401G in models of patient-derived cancer
BMC Cancer (2023)
-
Eribulin inhibits growth of cutaneous squamous cell carcinoma cell lines and a novel patient-derived xenograft
Scientific Reports (2023)
-
Cryopreservation of tissues by slow-freezing using an emerging zwitterionic cryoprotectant
Scientific Reports (2023)
-
Deep tumor-penetrating nano-delivery strategy to improve diagnosis and therapy in patient-derived xenograft (PDX) oral cancer model and patient tissue
Nano Research (2023)
-
Establishment and characterization of NCC-DSM1-C1: a novel cell line derived from a patient with desmoid fibromatosis
Human Cell (2023)