Abstract
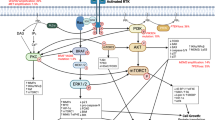
Glioblastomas are heterogeneous neoplasms that are driven by complex signalling pathways, and are among the most aggressive and challenging cancers to treat. Despite standard treatment with resection, radiation and chemotherapy, the prognosis of patients with glioblastomas remains poor. An increasing understanding of the molecular pathogenesis of glioblastomas has stimulated the development of novel therapies, including the use of molecular-targeted agents. Identification and validation of diagnostic, prognostic and predictive biomarkers has led to the advancement of clinical trial design, and identification of glioblastoma subgroups with a more-favourable prognosis and response to therapy. In this Review, we discuss common molecular alterations relevant to the biology of glioblastomas, targeted, antiangiogenic and immunotherapies that have impacted on the treatment of this disease, and the challenges and pitfalls associated with these therapies. In addition, we emphasize current biomarkers relevant to the management of patients with glioblastoma.
Key Points
-
Large-scale integrative genomic analyses have enhanced our current understanding of glioma biology and identified distinct tumour subtypes
-
Angiogenesis inhibitors and molecular-targeted agents are increasingly incorporated in the management of patients with glioma, although tumour resistance to existing therapies inevitably develops
-
Resistance mechanisms might be overcome by collective inhibition of multiple signalling pathways in selected patient populations
-
Novel immunotherapies in malignant gliomas have had promising results in early clinical trials
-
Several tissue biomarkers, including MGMT promoter methylation, IDH mutations and loss of 1p and 19q have become increasingly important in patient management and clinical trial design
-
Increasing real-time information about molecular and genetic alterations in patients with glioma, combined with the use of novel biomarkers, are likely to shape future therapies towards a more personalized medicine
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
CBTRUS. Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007 [online], (2011).
Ohgaki, H. & Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 64, 479–489 (2005).
Louis, D. N. et al. The World Health Organization classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Grossman, S. A. et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 16, 2443–2449 (2010).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
van den Bent, M. J. et al. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin. Cancer Res. 17, 7148–7155 (2011).
Cairncross, J. G. et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 90, 1473–1479 (1998).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Verhaak, R. G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17, 510–522 (2010).
Phillips, H. S. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006).
Huang, H. S. et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Biol. Chem. 272, 2927–2935 (1997).
Stommel, J. M. et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318, 287–290 (2007).
Snuderl, M. et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20, 810–817 (2011).
Szerlip, N. J. et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc. Natl Acad. Sci. USA 109, 3041–3046 (2012).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562 (2009).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3, 11–22 (2003).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8, 275–283 (2007).
Sidransky, D. et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature 355, 846–847 (1992).
Bögler, O., Huang, H. J. & Cavenee, W. K. Loss of wild-type p53 bestows a growth advantage on primary cortical astrocytes and facilitates their in vitro transformation. Cancer Res. 55, 2746–2751 (1995).
Reifenberger, G., Liu, L., Ichimura, K., Schmidt, E. E. & Collins, V. P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 53, 2736–2739 (1993).
Ohgaki, H. & Kleihues, P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 100, 2235–2241 (2009).
Stott, F. J. et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17, 5001–5014 (1998).
Norden, A. D., Drappatz, J. & Wen, P. Y. Antiangiogenic therapies for high-grade glioma. Nat. Rev. Neurol. 5, 610–620 (2009).
Gomez-Manzano, C. et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann. Neurol. 53, 109–117 (2003).
Yoshino, Y. et al. Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int. J. Oncol. 29, 981–987 (2006).
Kerbel, R. S. Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 (2008).
Jain, R. K. et al. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 8, 610–622 (2007).
Shih, Ie-M. & Wang, T. L. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 67, 1879–1882 (2007).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Dietrich, J., Imitola, J. & Kesari, S. Mechanisms of disease: the role of stem cells in the biology and treatment of gliomas. Nat. Clin. Pract. Oncol. 5, 393–404 (2008).
Dietrich, J., Diamond, E. L. & Kesari, S. Glioma stem cell signaling: therapeutic opportunities and challenges. Expert Rev. Anticancer Ther. 10, 709–722 (2010).
Hovinga, K. E. et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 28, 1019–1029 (2010).
Shen, Q. et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004).
Vescovi, A. L., Galli, R. & Reynolds, B. A. Brain tumour stem cells. Nat. Rev. Cancer 6, 425–436 (2006).
Jansen, M., Yip, S. & Louis, D. N. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol. 9, 717–726 (2010).
Reifenberger, G. & Louis, D. N. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J. Neuropathol. Exp. Neurol. 62, 111–126 (2003).
Burger, P. C. et al. Small cell architecture--a histological equivalent of EGFR amplification in glioblastoma multiforme? J. Neuropathol. Exp. Neurol. 60, 1099–1104 (2001).
Smith, J. S. et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J. Natl Cancer Inst. 93, 1246–1256 (2001).
Horbinski, C. et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 21, 564–574 (2011).
Camelo-Piragua, S. et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J. Neuropathol. Exp. Neurol. 70, 110–115 (2011).
Weller, M. et al. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin. Cancer Res. 13, 6933–6937 (2007).
Hartmann, C. et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 120, 707–718 (2010).
Wick, W. et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 13, 707–715 (2012).
van den Bent, M. J. et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J. Clin. Oncol. 27, 5881–5886 (2009).
Aldape, K., Burger, P. C. & Perry, A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Arch. Pathol. Lab. Med. 131, 242–251 (2007).
Wick, W. et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 27, 5874–5880 (2009).
Malmström, A. et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 13, 916–926 (2012).
Brandes, A. A. et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 26, 2192–2197 (2008).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Haas-Kogan, D. A. et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J. Natl Cancer Inst. 97, 880–887 (2005).
Mellinghoff, I. K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Brown, P. D. et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 26, 5603–5609 (2008).
Riemenschneider, M. J., Mueller, W., Betensky, R. A., Mohapatra, G. & Louis, D. N. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am. J. Pathol. 167, 1379–1387 (2005).
Chi, A. S. et al. Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal-epithelial transition inhibitor. J. Clin. Oncol. 30, e30–e33 (2012).
van den Bent, M. J. et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 27, 1268–1274 (2009).
Lassman, A. B. et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01–03 and 00–01 Clin. Cancer Res. 11, 7841–7850 (2005).
Neyns, B. et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol. 20, 1596–1603 (2009).
Thiessen, B. et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother. Pharmacol. 65, 353–361 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Galanis, E. et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 23, 5294–5304 (2005).
Kreisl, T. N. et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J. Neurooncol. 92, 99–105 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Wen, P. Y. et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin. Cancer Res. 12, 4899–4907 (2006).
Reardon, D. A. et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br. J. Cancer 101, 1995–2004 (2009).
Dresemann, G. et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J. Neurooncol. 96, 393–402 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Michaud, K. et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 70, 3228–3238 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Drappatz, J. et al. A phase I trial of LBH589 and bevacizumab for recurrent high-grade glioma (HGG) [abstract]. J. Clin. Oncol. 29 (Suppl.), a2050 (2011).
Galanis, E. et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J. Clin. Oncol. 27, 2052–2058 (2009).
Friday, B. B. et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol. 14, 215–221 (2012).
Phuphanich, S. et al. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J. Neurooncol. 100, 95–103 (2010).
Weller, M. et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 77, 1156–1164 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Liu, G. et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 5, 67 (2006).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Cohen, M. H., Shen, Y. L., Keegan, P. & Pazdur, R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14, 1131–1138 (2009).
Vredenburgh, J. J. et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 25, 4722–4729 (2007).
Friedman, H. S. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740 (2009).
Kreisl, T. N. et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27, 740–745 (2009).
Lai, A. et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 29, 142–148 (2011).
Sathornsumetee, S. et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 12, 1300–1310 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
de Groot, J. F. et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J. Clin. Oncol. 29, 2689–2695 (2011).
Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Batchelor, T. T. et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 28, 2817–2823 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Batchelor, T. et al. The efficacy of cediranib as monotherapy and in combination with lomustine compared to lomustine alone in patients with recurrent glioblastoma: a phase III randomized study. Neuro Oncol. 12 (Suppl. 4), 69–78 (2010).
Gerstner, E. et al. Effects of cediranib, a VEGF signaling inhibitor, in combination with chemoradiation on tumor blood flow and survival in newly diagnosed glioblastoma [abstract]. J. Clin. Oncol. 30 (Suppl.), a2009 (2012).
Wilhelm, S. et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 5, 835–844 (2006).
Hainsworth, J. D. et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer 116, 3663–3669 (2010).
Reardon, D. A. et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J. Neurooncol. 101, 57–66 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Wen, P. Y. American Society of Clinical Oncology 2010: report of selected studies from the CNS tumors section. Expert Rev. Anticancer Ther. 10, 1367–1369 (2010).
Neyns, B. et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J. Neurooncol. 103, 491–501 (2011).
Drappatz, J. et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 78, 85–90 (2010).
Quant, E. C. et al. Preliminary results from a multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma (GBM) [abstract]. J. Clin. Oncol. 29 (Suppl.), a2069 (2011).
Kreisl, T. N. et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 12, 181–189 (2010).
Avraamides, C. J., Garmy-Susini, B. & Varner, J. A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604–617 (2008).
Reardon, D. A. et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 26, 5610–5617 (2008).
Stupp, R. et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 2712–2718 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Dunn, G. P., Fecci, P. E. & Curry, W. T. Cancer immunoediting in malignant glioma. Neurosurgery 71, 201–222 (2012).
Cheever, M. A. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337 (2009).
Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 4722–4729 (2010).
Parsa, A. T. et al. A phase 2 multicenter trial of autologous heat shock protein peptide vaccine (HSPPC-96) for recurrent glioblastoma multiforme (GBM) patients shows improved survival compared to a contemporary cohort controlled for age, KPS and extent of resection [abstract]. J. Neurosurg. 117, A406 (2012).
Wheeler, C. J. & Black, K. L. DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin. Investig. Drugs 18, 509–519 (2009).
Wheeler, C. J. & Black, K. L. Vaccines for glioblastoma and high-grade glioma. Expert Rev. Vaccines 10, 875–886 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Fong, B. et al. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS ONE 7, e32614 (2012).
Phuphanich, S. et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. http://dx.doi.org/10.1007/s00262-012-1319-0.
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Ardon, H. et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. http://dx.doi.org/10.1007/s00262-012-1261-1.
Simoens, S. Pharmaco-economic aspects of Sipuleucel-T. Hum. Vaccin. Immunother. 8, 506–508 (2012).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Fecci, P. E. et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 13, 2158–2167 (2007).
Agarwalla, P., Barnard, Z., Fecci, P., Dranoff, G. & Curry, W. T. Jr. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J. Immunother. 35, 385–389 (2012).
Sunayama, J. et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells 28, 1930–1939 (2010).
Dias-Santagata, D. et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol. Med. 2, 146–158 (2010).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Weaver, K. D., Grossman, S. A. & Herman, J. G. Methylated tumor-specific DNA as a plasma biomarker in patients with glioma. Cancer Invest. 24, 35–40 (2006).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Schiff, D., Wen, P. Y. & van den Bent, M. J. Neurological adverse effects caused by cytotoxic and targeted therapies. Nat. Rev. Clin. Oncol. 6, 596–603 (2009).
Macdonald, D. R., Cascino, T. L., Schold, S. C. & Cairncross, J. G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 8, 1277–1280 (1990).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
Gállego Pérez-Larraya, J. et al. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 14, 667–673 (2012).
Norden, A. D. et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70, 779–787 (2008).
Iwamoto, F. M. et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73, 1200–1206 (2009).
Chamberlain, M. C. Radiographic patterns of relapse in glioblastoma. J. Neurooncol. 101, 319–323 (2011).
Wick, A. et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann. Neurol. 69, 586–592 (2011).
Sorensen, A. G., Batchelor, T. T., Wen, P. Y., Zhang, W. T. & Jain, R. K. Response criteria for glioma. Nat. Clin. Pract. Oncol. 5, 634–644 (2008).
Sorensen, A. G. et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 69, 5296–5300 (2009).
Gerstner, E. R. & Sorensen, A. G. Diffusion and diffusion tensor imaging in brain cancer. Semin. Radiat. Oncol. 21, 141–146 (2011).
Hu, L. S. et al. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 14, 919–930 (2012).
Ellingson, B. M. et al. Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro Oncol. 14, 333–343 (2012).
Andronesi, O. C. et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 4, 116ra4 (2012).
Quant, E. C. et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 11, 550–555 (2009).
Galanis, E. et al. Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol. 13, e196–e204 (2012).
Wen, P. Y. et al. It is time to include patients with brain tumors in phase I trials in oncology. J. Clin. Oncol. 29, 3211–3213 (2011).
Reardon, D. A. et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 13, 353–361 (2011).
Gilbert, M. R. Recurrent glioblastoma: a fresh look at current therapies and emerging novel approaches. Semin. Oncol. 38 (Suppl. 4), 21–33 (2011).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Brandes, A. A. et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J. Clin. Oncol. 24, 4746–4753 (2006).
Cairncross, J. G. et al. Chemotherapy plus radiotherapy (CT-RT) versus RT alone for patients with anaplastic oligodendroglioma: Long-term results of the RTOG 9402 phase III study [abstract]. J. Clin. Oncol. 30 (Suppl.), a2008b (2012).
Bauman, G. S. et al. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int. J. Radiat. Oncol. Biol. Phys. 48, 825–830 (2000).
van den Bent, M. J. et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J. Clin. Oncol. 24, 2715–2722 (2006).
Rivera, A. L. et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 12, 116–121 (2010).
Esteller, M. et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 343, 1350–1354 (2000).
Hegi, M. E. et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 26, 4189–4199 (2008).
Gerstner, E. R. et al. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology 73, 1509–1510 (2009).
Franceschi, E. et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br. J. Cancer 96, 1047–1051 (2007).
Acknowledgements
The authors gratefully acknowledge the support of the National Institute of Health (K24-CA125440, R01-CA129371, R01-CA117079-02, R01-CA57683), the American Academy of Neurology Foundation (J. Dietrich), the Stephen E. & Catherine Pappas Cancer Research Foundation (J. Dietrich), and gifts from the Montesi Family Research Fund and the Simches Fund for Brain Tumor Research. J. Dietrich is a fellow of the Clinical Investigator Training Program at Beth Israel Deaconess Medical Center, Harvard Medical School.
Author information
Authors and Affiliations
Contributions
S. Tanaka and J. Dietrich researched data for the article. All authors made a substantial contribution to the discussion of the content and to writing the manuscript. S. Tanaka, T. T. Batchelor, D. Louis and J. Dietrich edited and reviewed the article before submission.
Corresponding author
Ethics declarations
Competing interests
T. T. Batchelor acts as a consultant for Advance Medical, Champions Biotechnology, Kirin Pharmaceuticals, Merck & Co., Roche Pharmaceuticals and Spectrum Pharmaceuticals. In addition, he receives research support from AstraZeneca, Pfizer and Millennium. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Tanaka, S., Louis, D., Curry, W. et al. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end?. Nat Rev Clin Oncol 10, 14–26 (2013). https://doi.org/10.1038/nrclinonc.2012.204
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.204
This article is cited by
-
Identification of EMT-associated prognostic features among grade II/III gliomas
Scientific Reports (2024)
-
Inhibitory effects of temozolomide on glioma cells is sensitized by RSL3-induced ferroptosis but negatively correlated with expression of ferritin heavy chain 1 and ferritin light chain
Laboratory Investigation (2022)
-
Glioma Pericytes Promote Angiogenesis by Producing Periostin
Cellular and Molecular Neurobiology (2022)
-
EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma
Signal Transduction and Targeted Therapy (2022)
-
Comprehensive analysis of ceRNA network related to lincRNA in glioblastoma and prediction of clinical prognosis
BMC Cancer (2021)