Abstract
About one in 300 women will be diagnosed with breast cancer before the age of 40. Advances in screening have not had an impact on mortality in women who are too young to be candidates for screening. Risk factors for early breast cancer include a lean body habitus and recent use of an oral contraceptive. Breast cancers in very young women are typically aggressive, in part owing to the over-representation of high-grade, triple-negative tumours, but young age is an independent negative predictor of cancer-specific survival. Very early age-of-onset also correlates strongly with the risk of local recurrence and with the odds of contralateral breast cancer. Given the high risks of local and distant recurrence in young women with invasive breast cancer, most (if not all) young patients are candidates for chemotherapy. It is hoped that by increasing breast cancer awareness, the proportion of invasive breast cancers that are diagnosed at 2.0 cm or smaller will increase and that this will lead to a reduction in mortality.
Key Points
-
Rates of very early onset breast cancer have been stable in recent years
-
Early onset breast cancer is not clearly related to westernization or standard of living
-
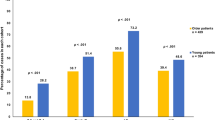
Young age is an independent risk factor for survival
-
Rates of ipsilateral recurrence and contralateral breast cancer are high in young women
-
Cancer control in young women should focus on breast cancer awareness
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ferlay, J. et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer [online], (2010).
Boyle, P. & Howell, A. The globalisation of breast cancer. Breast Cancer Res. 12 (Suppl. 4), S7 (2010).
Collins, L. C. et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res. Treat. 131, 1061–1066 (2012).
Voogd, A. C. et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J. Clin. Oncol. 19, 1688–1697 (2001).
Rubino, C., Arriagada, R., Delaloge, S. & Lê, M. G. Relation of risk of contralateral breast cancer to the interval since the first primary tumour. Br. J. Cancer 102, 213–219 (2010).
Bharat, A., Aft, R. L., Gao, F. & Margenthaler, J. A. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J. Surg. Oncol. 100, 248–251 (2009).
Ruddy, K. J. & Partridge, A. H. Breast cancer in young women: clinical decision-making in the face of uncertainty. Oncology (Williston Park) 23, 474, 477 (2009).
Azim, H. A. et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin. Cancer Res. 18, 13431–13453 (2012).
Central Intelligence Agency. The World Factbook [online], (2009).
Wasserman Schultz. The EARLY act [online], (2009).
Trichopoulos, D., Adami, H. O., Ekbom, A., Hsieh, C. C. & Lagiou, P. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int. J. Cancer. 122, 481–485 (2008).
Ahlgren, M. et al. Birth weight and risk of breast cancer in a cohort of 106,504 women. Int. J. Cancer 107, 997–1000 (2003).
Ahlgren, M., Melbye, M., Wohlfahrt, J. & Sørensen, T. I. Growth patterns and the risk of breast cancer in women. N. Engl. J. Med. 351, 1619–1626 (2004).
Trichopoulos, D. & Lipworth, L. Is cancer causation simpler than we thought, but more intractable? Epidemiology 6, 347–349 (1995).
Baik, I. et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 65, 358–363 (2005).
Kakarala, M. & Wicha, M. S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. 26, 2813–2820 (2008).
Narod, S. A. A model for breast cancer risk based on stem-cell theory. Curr. Oncol. 19, 9–11 (2012).
Hoover, R. N. et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 365, 1304–1314 (2011).
Bernstein, L., Henderson, B. E., Hanisch, R., Sullivan-Halley, J. & Ross, R. K. Physical exercise and reduced risk of breast cancer in young women. J. Natl Cancer Inst. 86, 1403–1408 (1994).
Gammon, M. D. et al. Recreational physical activity and breast cancer risk among women under age 45 years. Am. J. Epidemiol. 147, 273–280 (1998).
Rockhill, B. et al. Physical activity and breast cancer risk in a cohort of young women. J. Natl Cancer Inst. 90, 1155–1160 (1998).
Lahmann, P. H. et al. Physical activity and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomarkers Prev. 16, 36–42 (2007).
van Gils, C. H. et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA 293, 183–193 (2005).
Prentice, R. L. et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl Cancer Inst. 99, 1534–1543 (2007).
Martin, L. J. et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 71, 123–133 (2011).
Carmichael, A. R. & Bates, T. Obesity and breast cancer: a review of the literature. Breast 13, 85–92 (2004).
[No authors listed] Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 347, 1713–1727 (1996).
Althuis, M. D. et al. Breast cancers among very young premenopausal women. Cancer Causes Control 14, 151–160 (2003).
Clavel-Chapelon, F. & Gerber, M. Reproductive factors and breast cancer risk. Do they differ according to age of diagnosis. Breast Cancer Res. Treat. 72, 107–115 (2002).
Liu, Q. et al. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden). Cancer Causes Control 13, 299–305 (2002).
Lyons, T. R., Schedin, P. J. & Borges, V. F. Pregnancy and breast cancer: when they collide. J. Mammary Gland Biol. Neoplasia 14, 87–98 (2009).
[No authors listed] Breast feeding and risk of breast cancer in young women. United Kingdom National Case-Control Study Group. BMJ 307, 17–20 (1993).
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 360, 187–195 (2002).
[No authors listed] Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br. J. Cancer 83, 1301–1308 (2000).
Malone, K. E. et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer 88, 1393–1402 (2000).
Peto, J. et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J. Natl Cancer Inst. 91, 943–949 (1999).
Nichols, K. E., Malkin, D., Garber, J. E., Fraumeni, J. F. Jr & Li, F. P. Germ-line p53 mutations predispose to a wide spectrum of early-onset cancers. Cancer Epidemiol. Biomarkers Prev. 10, 83–87 (2001).
Malkin, D. et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250, 1233–1238 (1990).
Ginsburg, O. M. et al. The prevalence of germ-line TP53 mutations in women diagnosed with breast cancer before age 30. Familial Cancer 8, 563–567 (2009).
Mouchawar, J. et al. Population-based estimate of the contribution of TP53 mutations to subgroups of early-onset breast cancer; Australian Breast Cancer Family Study. Cancer Res. 70, 4795–4800 (2010).
Palmero, E. I. et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in Southern Brazil. Cancer Lett. 261, 21–25 (2008).
Gomes, M. C. et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered. Cancer Clin. Pract. 10, 3 (2012).
Zhang, S. et al. Frequency of the CHEK2 1100delC mutation among women with breast cancer: an international study. Cancer Res. 68, 2154–2157 (2008).
Narod, S. A. Testing for CHEK2 in the cancer genetics clinic: ready for prime time? Clin. Genet. 78, 1–7 (2010).
Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 358, 1389–1399 (2001).
Ronckers, C. M., Erdmann, C. A. & Land, C. E. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 7, 21–32 (2005).
Kenney, L. B. et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann. Intern. Med. 141, 590–597 (2004).
van Leeuwen, F. E. et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J. Natl Cancer Inst. 95, 971–980 (2003).
Travis, L. B. et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J. Natl Cancer Inst. 97, 1428–1437 (2005).
Land, C. E. et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990 Radiat. Res. 160, 707–717 (2003).
Land, C. E. et al. A case-control interview study of breast cancer among Japanese A-bomb survivors. II. Interactions with radiation dose. Cancer Causes Control 5, 167–176 (1994).
Kerlikowske, K. Epidemiology of ductal carcinoma in situ. J. Natl Cancer Inst. Monogr. 41, 139–141 (2010).
Lund, M. J. et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer 116, 2549–2559 (2010).
Anders, C. K. et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J. Clin. Oncol. 26, 3324–3330 (2008).
Kroman, N. et al. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ 320, 474–478 (2000).
Nixon, A. J. et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J. Clin. Oncol. 12, 888–894 (1994).
Gnerlich, J. L. et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J. Am. Coll. Surg. 208, 341–347 (2009).
Xiong, Q. et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis: the MD Anderson Cancer Center experience. Cancer 92, 2523–2528 (2001).
Narod, S. A. Age of diagnosis, tumor size, and survival after breast cancer: implications for mammographic screening. Breast Cancer Res. Treat. 128, 259–266 (2011).
de Bock, G. H. et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur. J. Cancer 42, 351–356 (2006).
Wapnir, I. L. et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J. Clin. Oncol. 24, 2028–2037 (2006).
Fourquet, A. et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int. J. Radiat. Oncol. Biol. Phys. 17, 719–725 (1989).
Van der Leij, F. et al. Predictive factors for local recurrence in breast cancer. Semin. Radiat. Oncol. 22, 100–107 (2012).
Hartman, M. et al. Genetic implications of bilateral breast cancer: a population based cohort study. Lancet Oncol. 6, 377–382 (2005).
Boyd, N. F. et al. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr. Oncol. Rep. 3, 314–321 (2001).
Cil, T. et al. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer 115, 5780–5787 (2009).
Checka, C. M., Chun, J. E., Schnabel, F. R., Lee, J. & Toth, H. The relationship of mammographic density and age: implications for breast cancer screening. Am. J. Roentgenol. 198, W292–W295 (2012).
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 (2008).
Narod, S. A. Early-onset breast cancer: what do we know about the risk factors?: A Countercurrents Series. Curr. Oncol. 18, 204–205 (2011).
Beck, A. H. et al. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci. Transl. Med. 3, 108 (2011).
Metcalfe, K. et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 22, 2328–2335 (2004).
Peralta, E. A. et al. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am. J. Surg. 180, 439–445 (2000).
Bedrosian, I., Hu, C. Y. & Chang, G. J. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J. Natl Cancer Inst. 102, 401–409 (2010).
Narod, S. A. The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Res. Treat. 128, 581–583 (2011).
Berek, J. S. et al. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer. Obstet. Gynecol. 116, 733–743 (2010).
Early Breast Cancer Trialists' Collaborative Group (EBCTCG) et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379, 432–444 (2012).
Kroman, N. et al. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ 320, 474–478 (2000).
Robson, M. E. et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 6, R8–R17 (2004).
Rennert, G. et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 357, 115–123 (2007).
Goffin, J. R. et al. Impact of germline BRCA1 mutations and overexpression of p53 on prognosis and response to treatment following breast carcinoma: 10-year follow up data. Cancer 97, 527–536 (2003).
Goldhirsch, A. et al. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J. Natl Cancer Inst. 87, 1441–1445 (1995).
Freedman, R. A. & Partridge, A. H. Adjuvant therapies for very young women with early stage breast cancer. Breast 20 (Suppl. 3), S146–S149 (2011).
[No authors listed] Polychemotherapy for early breast cancer: an overview of randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 352, 930–942 (1998).
Jakesz, R. et al. Austrian Breast and Colorectal Cancer Study Group Trial 5. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J. Clin. Oncol. 20, 4621–4627 (2002).
Thomas, D. B. et al. Randomized trial of breast self-examination in Shanghai: final results. J. Natl Cancer Inst. 94, 1445–1457 (2002).
Ogawa, H., Tominaga, S., Yoshida, M., Kubo, K. & Takeuchi, S. Breast self-examination practice and clinical stage of breast cancer. Jpn J. Cancer Res. 78, 447–452 (1987).
Koibuchi, Y. et al. The effect of mass screening by physical examination combined with regular breast self-examination on clinical stage and course of Japanese women with breast cancer. Oncol. Rep. 5, 151–155 (1998).
Port, E. R., Montgomery, L. L., Heerdt, A. S. & Borgen P. I. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann. Surg. Oncol. 8, 580–585 (2001).
Julian-Reynier, C. M. et al. Women's attitudes toward preventive strategies for hereditary breast or ovarian carcinoma differ from one country to another: differences among English, French, and Canadian women. Cancer 92, 959–968 (2001).
Metcalfe, K. A. et al. Hereditary Breast Cancer Clinical Study Group. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer. 122, 2017–2022 (2008).
Iqbal, J. et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: A systematic review. Cancer Treat. Rev. 38, 318–328 (2012).
Surveillance, Epidemiology and End Results. 1973-2008 Data (Nov 2010 Submission) [online], (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Narod, S. Breast cancer in young women. Nat Rev Clin Oncol 9, 460–470 (2012). https://doi.org/10.1038/nrclinonc.2012.102
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.102
This article is cited by
-
Increased risk of contralateral breast cancer for BRCA1/2 wild-type, high-risk Korean breast cancer patients: a retrospective cohort study
Breast Cancer Research (2024)
-
Incidence trends for twelve cancers in younger adults—a rapid review
British Journal of Cancer (2022)
-
Risk factors for psychological morbidity and the protective role of coping self-efficacy in young women with breast cancer early in diagnosis: a national multicentre cohort study
Breast Cancer Research and Treatment (2022)
-
Oncobiology and treatment of breast cancer in young women
Cancer and Metastasis Reviews (2022)
-
Clinical features and prognostic factors of breast cancer in young women: a retrospective single-center study
Archives of Gynecology and Obstetrics (2022)