Abstract
Esophagogastric cancer represents a significant global health problem, with most patients presenting with advanced-stage disease and consequently with a poor prognosis. Chemotherapy improves survival compared with supportive care alone, and combination chemotherapy regimens are more effective than monotherapy. Overexpression of EGFR and possibly HER2 confer a poor prognosis, providing potentially important therapeutic targets for selected patients. Inhibition of HER2 with the monoclonal antibody trastuzumab in patients with HER2 overexpression, HER2 gene amplification, or both, is effective and has been the standard of care for HER2-positive breast cancer for almost a decade. In patients with advanced-stage gastric or esophagogastric-junction adenocarcinomas, the addition of trastuzumab to a cisplatin plus fluoropyrimidine doublet was reported to improve response rate, progression-free survival and overall survival, with the greatest benefit reported in the subgroup of patients with the highest expression of HER2. Cetuximab and panitumumab, two monoclonal antibodies against EGFR, and the dual EGFR and HER2 tyrosine kinase inhibitor lapatinib are currently undergoing phase III evaluation in esophagogastric cancer. We discuss the preclinical rationale for targeting human EGFRs and recent clinical reports of these targeted agents in esophagogastric cancer.
Key Points
-
EGFR overexpression is a common finding in advanced-stage esophagogastric cancer and correlates with poor prognosis
-
Early efficacy data from phase II studies indicate that inhibition of EGFR using monoclonal antibodies in combination with chemotherapy might be beneficial in advanced-stage esophagogastric cancer
-
KRAS and EGFR mutations are established biomarkers for use of anti-EGFR antibodies and tyrosine kinase inhibitors in colorectal and lung cancer, respectively, but their role in esophagogastric cancer remains undefined
-
Phase III data supporting combination of anti-EGFR antibodies or tyrosine kinase inhibitors with neoadjuvant chemoradiotherapy for localized esophageal cancer are still lacking—therefore, this combination is not a standard approach
-
HER2 overexpression is most commonly seen in intestinal-type esophagogastric cancer and might correlate with a poor prognosis
-
The combination of trastuzumab with chemotherapy improves response rate and survival of patients with advanced-stage gastric or esophagogastric-junction cancer and represents a new standard of care
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 (2010).
Macdonald, J. S. et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 345, 725–730 (2001).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophagael cancer. N. Engl. J. Med. 355, 11–20 (2006).
Wagner, A. D. et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews, Issue 3. Art. No.: CD004064. doi:10.1002/14651858.CD004064.pub3 (2010).
Cunningham, D. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 358, 36–46 (2008).
Kang, Y. K. et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann. Oncol. 20, 666–673 (2009).
Koizumi, W. et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 9, 215–221 (2008).
Van Cutsem, E. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24, 4991–4997 (2006).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
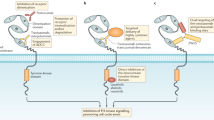
Marshall, J. Clinical implications of the mechanism of epidermal growth factor receptor inhibitors. Cancer 107, 1207–1218 (2006).
Harari, D. & Yarden, Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 19, 6102–6114 (2000).
Pinkas-Kramarski, R. et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 15, 2452–2467 (1996).
Karamouzis, M. V., Badra, F. A. & Papavassiliou, A. G. Breast cancer: the upgraded role of HER-3 and HER-4. Int. J. Biochem. Cell Biol. 39, 851–856 (2007).
Kim, M. A. et al. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 52, 738–746 (2008).
Galizia, G. et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J. Surg. 31, 1458–1468 (2007).
Lieto, E. et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann. Surg. Oncol. 15, 69–79 (2008).
Moutinho, C. et al. Epidermal growth factor receptor structural alterations in gastric cancer. BMC Cancer 8, 10 (2008).
Wang, K. L. et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer 109, 658–667 (2007).
Langer, R. et al. Prognostic significance of expression patterns of c-erbB-2, p53, p16INK14A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J. Clin. Pathol. 59, 631–634 (2006).
Itakura, Y. et al. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer 74, 795–804 (1994).
Hanawa, M. et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int. J. Cancer 118, 1173–1180 (2006).
Kitagawa, Y. et al. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin. Cancer Res. 2, 909–914 (1996).
Kwak, E. L. et al. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin. Cancer Res. 12, 4283–4287 (2006).
Spector, N. L. & Blackwell, K. L. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 27, 5838–5847 (2009).
Enzinger, P. C. et al. CALGB 80403/ECOG 1206: a randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a4006 (2010).
Chan, J. A. et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann. Oncol. doi:10.1093/annonc/mdq604.
Gold, P. J. et al. Cetuximab as second-line therapy in patients with metastatic esophageal adenocarcinoma: a phase II Southwest Oncology Group Study (S0415). J. Thorac. Oncol. 5, 1472–1476 (2010).
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Lorenzen, S. et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann. Oncol. 20, 1667–1673 (2009).
Sgroi, M. M. et al. Preoperative cetuximab and radiation (XRT) for patients (pts) with surgically resectable esophageal and gastroesophageal (GE) junction carcinomas: A pilot study from the Hoosier Oncology Group and the University of Texas-Southwestern [abstract]. J. Clin. Oncol. 26 (15 Suppl.), a4564 (2008).
Enzinger, P. C. et al. Phase II cisplatin, irinotecan, cetuximab and concurrent radiation therapy followed by surgery for locally advanced esophageal cancer [abstract]. J. Clin. Oncol. 24 (18 Suppl.), a4064 (2006).
Lledo, G. et al. Chemoradiation with FOLFOX plus cetuximab in stage III cardia or esophageal cancer: Interim analysis from a GERCOR phase II trial (ERaFOX) [abstract]. ASCO Gastrointestinal Symp. a68 (2010).
De Vita, F. et al. A multicenter phase II study of induction chemotherapy with FOLFOX-4 and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. Br. J. Cancer 104, 427–432 (2011).
Yu, J. M. et al. An open label, multicenter clinical study of cetuximab combined with concurrent chemoradiotherapy for locally advanced esophageal squamous cell carcinoma: Preliminary results of a phase II trial [abstract]. J. Clin. Oncol. 28 (15 Suppl.), e14520 (2010).
Safran, H. et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int. J. Radiat. Oncol. Biol. Phys. 70, 391–395 (2008).
Wanebo, H. J., DiPetrillo, T. & Kennedy, T. Cetuximab-based neoadjuvant chemoradiation appears to facilitate surgical resection in patients with locally advanced esophageal and gastric cancer [abstract]. ASCO Gastrointestinal Cancers Symp. a33 (2008).
Walsh, T. N. et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N. Engl. J. Med. 335, 462–467 (1996).
Tepper, J. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092 (2008).
Ruhstaller, T. et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II trial (SAKK 75/06). J. Clin. Oncol. 29, 626–631 (2011).
Rao, S. et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann. Oncol. 21, 2213–2219 (2010).
Rao, S. et al. Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br. J. Cancer 99, 868–874 (2008).
Okines, A. F. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J. Clin. Oncol. 28, 3945–3950 (2010).
Adams, R. A. et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br. J. Cancer 100, 251–258 (2009).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Allgayer, H. et al. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J. Clin. Oncol. 18, 2201–2209 (2000).
Tanner, M. et al. Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 16, 273–278 (2005).
Grabsch, H., Sivakumar, S., Gray, S., Gabbert, H. E. & Muller, W. HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell. Oncol. 32, 57–65 (2010).
Ohguri, T., Sato, Y., Koizumi, W., Saigenji, K. & Kameya, T. An immunohistochemical study of c-erbB-2 protein in gastric carcinomas and lymph-node metastases: is the c-erbB-2 protein really a prognostic indicator? Int. J. Cancer 53, 75–79 (1993).
Bang, Y. et al. Pathological features of advanced gastric cancer (GC): relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial [abstract]. J. Clin. Oncol. 27 (15 Suppl.), a4556 (2009).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52, 797–805 (2008).
Herceptin®(Genentech, Inc., San Francisco, CA) (trastuzumab) prescribing information [online]. (2010).
Chau, I. et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma—individual patient data from 1775 patients in four randomised controlled trials. Ann. Oncol. 20, 885–891 (2009).
Doi, T. et al. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer [abstract]. Proc. Am. Soc. Clin. Oncol. 22, a1036 (2003).
Dragovich, T. et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J. Clin. Oncol. 24, 4922–4927 (2006).
US National Library of Medicine. Clinicaltrials.gov [online]. (2011).
Taira, N. et al. Gefitinib, an epidermal growth factor receptor blockade agent, shows additional or synergistic effects on the radiosensitivity of esophageal cancer cells in vitro. Acta Med. Okayama 60, 25–34 (2006).
Javle, M. et al. Pilot study of gefitinib, oxaliplatin, and radiotherapy for esophageal adenocarcinoma: tissue effect predicts clinical response. Am. J. Clin. Oncol. 31, 329–334 (2008).
Rodriguez, C. P. et al. A phase II study of perioperative concurrent chemotherapy, gefitinib, and hyperfractionated radiation followed by maintenance gefitinib in locoregionally advanced esophagus and gastroesophageal junction cancer. J. Thorac. Oncol. 5, 229–235 (2010).
Li, G. et al. Phase II study of concurrent chemoradiation in combination with erlotinib for locally advanced esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 78, 1407–1412 (2010).
Meluch, A. A. et al. A phase II trial of preoperative chemoradiation therapy plus bevacizumab and erlotinib in the treatment of localized esophageal cancer [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a4108 (2010).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Tsao, M. S. et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N. Engl. J. Med. 353, 133–144 (2005).
Kim, J. W. et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 272, 296–306 (2008).
LaBonte, M. J. et al. The dual EGFR/HER-2 tyrosine kinase inhibitor lapatinib sensitizes colon and gastric cancer cells to the irinotecan active metabolite SN-38. Int. J. Cancer 125, 2957–2969 (2009).
Tanizaki, J. et al. Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol. Cancer Ther. 9, 1198–1207 (2010).
Wainberg, Z. A. et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin. Cancer Res. 16, 1509–1519 (2010).
Iqbal, S. et al. S0413: a phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients (pts) with advanced or metastatic gastric cancer [abstract]. J. Clin. Oncol. 25 (18 Suppl.), a4621 (2007).
Satoh, T. et al. Interim safety analysis from TYTAN: a phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a4057 (2010).
Hecht, J. et al. A phase III study of CapeOx ± lapatinib in HER2 positive locally-advanced/metastatic upper gastrointestinal adenocarcinoma: interim safety results [abstract]. Eur. J. Cancer Suppl. 7, 385 (2009).
Gonzales, A. J. et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol. Cancer Ther. 7, 1880–1889 (2008).
Engelman, J. A. et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 67, 11924–11932 (2007).
Mok, T. et al. Efficacy and safety of PF-00299804 (PF299), an oral, irreversible, pan-human epidermal growth factor receptor (pan-HER) tyrosine kinase inhibitor (TKI), as first-line treatment (tx) of selected patients (pts) with advanced (adv) non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a7537 (2010).
Baselga, J. & Swain, S. M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 9, 463–475 (2009).
Hayashi, M. et al. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin. Cancer Res. 14, 7843–7849 (2008).
Sanidas, E. E. et al. Expression of the c-erbB-3 gene product in gastric cancer. Int. J. Cancer 54, 935–940 (1993).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Burris, H. A. 3rd et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2011).
Gelmon, K. A. et al. Results of a phase II trial of trastuzumab (H) and pertuzumab (P) in patients (pts) with HER2-positive metastatic breast cancer (MBC) who had progressed during trastuzumab therapy [abstract]. J. Clin. Oncol. 26 (15 Suppl.), a1026 (2008).
Miller, K. et al. A phase Ib/II trial of trastuzumab-DM1 (T-DM1) with pertuzumab (P) for women with HER2-positive, locally advanced or metastatic breast cancer (BC) who were previously treated with trastuzumab (T) [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a1012 (2010).
Jones, F. E., Welte, T., Fu, X-Y. & Stern, D. F. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell Biol. 147, 77–87 (1999).
Junttila, T. T. et al., Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 65, 1384–1393 (2005).
Ott, K. et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J. Clin. Oncol. 24, 4692–4698 (2006).
Lordick, F. et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 8, 797–805 (2007).
Pinto, C. et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann. Oncol. 18, 510–517 (2007).
Di Fabio, F. et al. The predictive value of 18F-FDG-PET early evaluation in patients with metastatic gastric adenocarcinoma treated with chemotherapy plus cetuximab. Gastric Cancer 10, 221–227 (2007).
Maughan, T. S. et al. Identification of potentially responsive subsets when cetuximab is added to oxaliplatin-fluoropyrimidine chemotherapy (CT) in first-line advanced colorectal cancer (aCRC): mature results of the MRC COIN trial [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a3502 (2010).
Alberts, S. R. et al. Adjuvant mFOLFOX6 with or without cetuxiumab (Cmab) in KRAS wild-type (WT) patients (pts) with resected stage III colon cancer (CC): results from NCCTG Intergroup Phase III Trial N0147 [abstract]. J. Clin. Oncol. 28 (18 Suppl.), CRA3507 (2010).
Lynch, T. J. et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J. Clin. Oncol. 28, 911–917 (2010).
Khambata-Ford, S. et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 918–927 (2010).
Stella, G. et al. KRAS and BRAF mutational status as response biomarkers to cetuximab combination therapy in advanced gastric cancer patients [abstract]. J. Clin. Oncol. 27 (15 Suppl.), e15503 (2009).
Lord, V. N., O'Grady, R., Sheehan, C., Field, A. F. & Ward, R. L. K-ras codon 12 mutations in Barrett's oesophagus and adenocarcinomas of the oesophagus and oesophagogastric junction. J. Gastroenterol. Hepatol. 15, 730–736 (2000).
De Roock, W. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010).
Sommerer, F. et al. Mutations of BRAF and KRAS2 in the development of Barrett's adenocarcinoma. Oncogene 23, 554–558 (2004).
Velho, S. et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur. J. Cancer 41, 1649–1654 (2005).
Phillips, W. A. et al. Mutation analysis of PIK3CA and PIK3CB in esophageal cancer and Barrett's esophagus. Int. J. Cancer 118, 2644–2646 (2006).
Im, S. A. et al. Potential prognostic significance of p185(HER2) overexpression with loss of PTEN expression in gastric carcinomas. Tumori 91, 513–521 (2005).
Laurent-Puig, P. et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 27, 5924–5930 (2009).
Jacobs, B. et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J. Clin. Oncol. 27, 5068–5074 (2009).
Wu, W. K. et al. Expression of ErbB receptors and their cognate ligands in gastric and colon cancer cell lines. Anticancer Res. 29, 229–234 (2009).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Hecht, J. R. et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 110, 980–988 (2007).
Sartore-Bianchi, A. et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J. Clin. Oncol. 25, 3238–3245 (2007).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Pinto, C. et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br. J. Cancer 101, 1261–1268 (2009).
Lordick, F. et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br. J. Cancer 102, 500–505 (2010).
Kim, C. et al. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest. New Drugs 29, 366–373 (2009).
Han, S. W. et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br. J. Cancer 100, 298–304 (2009).
Ferry, D. R. et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin. Cancer Res. 13, 5869–5875 (2007).
Janmaat, M. L. et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J. Clin. Oncol. 24, 1612–1619 (2006).
Adelstein, D. J., Rybicki, L. A., Carroll, M. A., Rice, T. W. & Mekhail, T. Phase II trial of gefitinib for recurrent or metastatic esophageal or gastroesophageal junction (GEJ) cancer [abstract]. J. Clin. Oncol. 23 (16 Suppl.), a4054 (2005).
Ilson, D. H. et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer doi:10.1002/cncr.25602 (2010).
Acknowledgements
We acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Center.
Author information
Authors and Affiliations
Contributions
A. Okines and I. Chau researched the data for the article, wrote the article and provided a substantial contribution to discussion of the content. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A. Okines declares that she received grant/research support from Roche, Amgen, and Bayer. D. Cunningham declares that he is a consultant and and received grant/research support from Roche, Amgen, and Merck. I. Chau declares that he is consultant for Roche, Merck Serono, Novartis, ImClone, and OSI. He is on the speaker's bureau (honoraria) for Roche and Sanofi-Aventis, and received grant/research support from Merck Serono and Novartis.
Rights and permissions
About this article
Cite this article
Okines, A., Cunningham, D. & Chau, I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 8, 492–503 (2011). https://doi.org/10.1038/nrclinonc.2011.45
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2011.45
This article is cited by
-
Bromodomain protein BRDT directs ΔNp63 function and super-enhancer activity in a subset of esophageal squamous cell carcinomas
Cell Death & Differentiation (2021)
-
Cetuximab for esophageal cancer: an updated meta-analysis of randomized controlled trials
BMC Cancer (2018)
-
Her2 Ile655Val polymorphism and its association with breast cancer risk: an updated meta-analysis of case-control studies
Scientific Reports (2018)
-
Lapatinib in combination with paclitaxel plays synergistic antitumor effects on esophageal squamous cancer
Cancer Chemotherapy and Pharmacology (2018)
-
Anti-tubulin drugs conjugated to anti-ErbB antibodies selectively radiosensitize
Nature Communications (2016)