Abstract
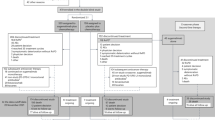
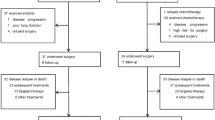
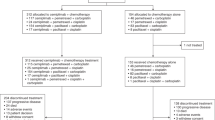
The first phase III study to assess the effect of gefitinib and docetaxel on the survival of Japanese patients with non-small-cell lung cancer who received previous treatment with platinum doublets, the V15-32 trial, did not establish noninferiority of gefitinib over docetaxel in terms of the effect on overall survival, despite the results showing a twofold higher response rate to gefitinib. The overall survival favored docetaxel for the first 18 months and gefitinib thereafter. The INTEREST trial, which compared docetaxel and gefitinib, demonstrated noninferiority of gefitinib, and the survival curves were completely superimposed. In this trial, patients had been recruited from 24 countries from Europe, Asia, and North and South America. Results of the IPASS trial showed superior progression-free survival for gefitinib compared with the combination of carboplatin and paclitaxel as first-line treatment in Asian patients who were nonsmokers and had adenocarcinoma histology. In this Review, we discuss the reasons for the differences in the effects of molecular-targeted drugs and cytotoxic antineoplastic agents observed in these trials. We also highlight the magnitude of the antitumor activity of these two different categories of drugs, and discuss how this could affect future clinical trial design and analysis.
Key Points
-
Many unexpected results were observed in the randomized, controlled trials of EGFR-targeted tyrosine kinase inhibitors (TKIs)
-
The nature and quantity of antitumor effects are different between cytotoxic chemotherapy and molecular-targeted drugs
-
Selection of patients is extremely important for future clinical trials that test EGFR-TKIs
-
Results from the IPASS trial demonstrate that EGFR-TKIs provide superior progression-free survival compared with platinum-based doublet chemotherapy in selected patients with non-small-cell lung cancer, especially those with mutated EGFR
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schiller, J. H. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 346, 92–98 (2002).
Ohe, Y. et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann. Oncol. 18, 317–323 (2007).
Kelly, K. et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J. Clin. Oncol. 19, 3210–3218 (2001).
Fossella, F. et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J. Clin. Oncol. 21, 3016–3024 (2003).
Hynes, N. E. & Lane, H. A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 (2005).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Ranson, M. et al. ZD1849, a selective oral epidermoid growth factor receptor tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J. Clin. Oncol. 20, 2240–2250 (2002).
Herbst, R. S. et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J. Clin. Oncol. 20, 3815–3825 (2002).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Gatzemeier, U. et al.: Results of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small-cell lung cancer (NSCLC) [Abstract]. ASCO Meeting Abstracts 22, 7010 (2004).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Herbst, R. S. et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 23, 5892–5899 (2005).
Kelly, K. et al. Updated analysis of SWOG 0023: A randomized phase III trial of gefitinib versus placebo maintenance after definitive chemoradiation followed by docetaxel in patients with locally advanced stage III non-small-cell lung cancer [Abstract]. ASCO Meeting Abstracts 25, 7513 (2007).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Han, S. W. et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell-lung cancer patients treated with gefitinib. J. Clin. Oncol. 23, 2493–2501 (2005).
Fukuoka, M. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J. Clin. Oncol. 21, 2237–2246 (2003).
Chang, A. et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL study. J. Thorac. Oncol. 1, 847–855 (2006).
Niho, S. et al. First-line single agent of gefitinib in patients with advanced non-small-cell lung cancer: a phase II study. J. Clin. Oncol. 24, 64–69 (2003).
Lee, D. H. et al. Gefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokers. Clin. Cancer Res. 11, 3032–3037 (2005).
Shigematsu, H. et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl Cancer Inst. 97, 339–346 (2005).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Johnson, B. E. & Jänne, P. A. Selecting patients for epidermal growth factor receptor inhibitor treatment: A FISH story or a tale of mutations? J. Clin. Oncol. 23, 6813–6816 (2005).
Sequist, L. V. et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist 12, 90–98 (2007).
Marchetti, A. et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 23, 857–865 (2005).
Mitsudomi, T. et al. Mutations of the epidermal growth factor receptor gene predicts prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J. Clin. Oncol. 23, 2513–2520 (2005).
Saijo, N. Advances in the treatment of non-small-cell lung cancer. Cancer Treat. Rev. 34, 521–526 (2008).
Inoue, A. et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small cell lung cancer with epidermal growth factor receptor gene mutations. J. Clin. Oncol. 2, 3340–3346 (2006).
Takano, T. et al. Epidermal growth factor receptor gene mutations and increased copy members predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 23, 6829–6837 (2005).
Mitsudomi, T. & Yatabe, Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 98, 1817–1824 (2007).
Yoshida, K. et al., Comparison of the efficacy between chemotherapy and gefitinib as 1st line setting in patients with EGFR mutation positive NSCLC [Abstract 278P]. Ann. Oncol. 19 (Suppl. 8) viii104 (2008).
Cappuzzo, F. et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl Cancer Inst. 97, 643–655 (2005).
Hirsch, F. R. et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J. Clin. Oncol. 23, 6838–6845 (2005).
Tsao, M. S. et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N. Engl. J. Med. 23, 133–144 (2005).
Fossella, F. V. et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small-Cell Lung Cancer Study Group. J. Clin. Oncol. 18, 2354–2362 (2000).
Shepherd, F. A. et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J. Clin. Oncol. 18, 2095–2103 (2000).
Hanna, N. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 22, 1589–1597 (2004).
Ohe, Y. et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small-cell lung cancer. Clin. Cancer Res. 14, 4206–4212 (2008).
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Tamura, T. et al. Evaluation of efficacy and safety of erlotinib as monotherapy for Japanese patients with advanced non-small-cell lung cancer; Integrated analysis of two Japanese phase II studies [Abstract]. J. Thorac. Oncol. 2 (Suppl. 4), S742 (2007).
Maruyama, R. et al. Phase III study, V-15-32 of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J. Clin. Oncol. 26, 4244–4252 (2008).
Takeuchi, M. Another statistical analysis on the survival rate at various time intervals in patients accrued to V15–32 studied. Drug Safety Policy Panel, Safety Investigation committee, Second Food and Drug Advisory Board of February 1 2007 (2006).
Kunitoh, H. Critical comments on V15–32 study, The 5th Annual Meeting of Japanese Society of Medical Oncology PS-15, (2007).
Fushimi, T. Drug Safety Policy Panel, Safety Policy Investigation Committee, Second Food and Drug Advisory Board of Ministry of health Labor and Welfare, February 1, 2007 (2006).
Kim, E. S. et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372, 1809–1818 (2008).
Mok, T. et al. Phase III, randomized, open-label, first line study of gefitinib vs carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer (NSCLC) (IPASS) [Abstract LBA2]. Ann. Oncol. 19 (Suppl. 8), viii1 (2008).
Tsuboi, M. et al. Gefitinib in the adjuvant setting: safety results from a phase III study in patients with completely resected non-small-cell lung cancer. Anticancer Drugs 16, 1123–1128 (2005).
Giaccone, G. & Rodriguez, J. A. EGFR inhibitors: what have we learned from the treatment of lung cancer? Nat. Clin. Pract. Oncol. 2, 554–561 (2005).
Crino, L. et al. Gefitinib (IRESSA) versus vinorelbine in chemonaive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized phase II study: B3–04. J. Thorac. Oncol. 2 (Suppl. 4), S341 (2007).
Saijo, N. Recent trends in the treatment of advanced lung cancer. Cancer Sci. 97, 448–452 (2006).
Lilenbaum, R. et al. Randomized phase II trial of single agent erlotinib vs. standard chemotherapy in patients with advanced non-small-cell lung cancer (NSCLC) and performance status (PS) of 2 [Abstract]. ASCO Meeting Abstracts. 24, 7022 (2006).
Hida, I et al. Randomized phase III study of platinum-doublet chemotherapy followed by gefitinib versus continued platinum-doublet chemotherapy in patients with advanced non-small-cell lung cancer (NSCLC): Results of West Japan Thoracic Oncology Group trial (WJTOG) [Abstract]. ASCO Meeting Abstracts 26, LBA8012 (2008).
Acknowledgements
The authors would like to acknowledge the effort of the investigators of AstraZeneca's trials such as the V15-32 INTEREST and IPASS trials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N. Saijo declared he receives research support from AstraZeneca, Bristol–Myers Squibb, and Chugai–Roche. He is also on the speakers bureau for Eli Lilly. H. Kunitoh declared he is on the speakers bureau for AstraZeneca, Bristol–Myers Squibb and Sanofi–Aventis. H. Takeuchi declared no competing interests.
Rights and permissions
About this article
Cite this article
Saijo, N., Takeuchi, M. & Kunitoh, H. Reasons for response differences seen in the V15-32, INTEREST and IPASS trials. Nat Rev Clin Oncol 6, 287–294 (2009). https://doi.org/10.1038/nrclinonc.2009.37
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.37