Abstract
Trastuzumab is a monoclonal antibody directed against the human EGFR2 (HER2) protein that has been shown to improve survival in patients with HER2-positive breast cancer. Lapatinib is an oral small-molecule tyrosine kinase inhibitor directed against EGFR and HER2. Lapatinib therapy was shown to prolong the time to progression and increase the rate of response to capecitabine in patients who had received anthracycline-based and taxane-based chemotherapy, and whose tumors had progressed on trastuzumab. HER2 status, either gene copy number or the protein expression level, is the best predictive marker available for assessing response to trastuzumab and lapatinib. Whether the power of this predictive marker is the same in advanced and early-stage cancers is unknown. There is great interest in developing diagnostic tests that predict which patients are more likely to benefit from specific HER2-directed therapies. Novel therapeutics that will overcome resistance to trastuzumab and lapatinib are under intense clinical development. In the future, it will be important to characterize mechanisms of resistance in metastatic tumors to determine which novel targeted therapy will be most appropriate for individual patients.
Key Points
-
The HER2 status of all invasive breast cancers should be assessed by immunohistochemistry (IHC) or in situ hybridization; the former method is most commonly used worldwide
-
Trastuzumab and lapatinib are effective therapies in HER2-positive breast cancer; however, not all HER2-positive patients respond to these therapies, and progression is common in responding patients with metastatic disease
-
Potential molecular predictors of resistance to HER2-directed therapies include loss of PTEN, PI3K/Akt hyperactivation, p95HER2 expression, and IGF-IR overexpression
-
Novel therapeutics are in clinical development to overcome resistance to trastuzumab and lapatinib, and include pertuzumab, trastuzumab-DM1, PI3K inhibitors, HSP90 inhibitors, IGF-IR inhibitors and novel tyrosine kinase inhibitors
-
Randomized clinical trials have shown an improved clinical benefit in patients with HER2-positive metastatic disease who have disease progression and have been treated with drugs that inhibit HER2
-
The molecular mechanism of HER2 blockade beyond disease progression is not known, and represents a new paradigm in breast cancer therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
King, C. R., Kraus, M. H. & Aaronson, S. A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 229, 974–976 (1985).
Di Fiore, P. P. et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 51, 1063–1070 (1987).
Finkle, D. et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin. Cancer Res. 10, 2499–2511 (2004).
Drebin, J. A., Link, V. C., Stern, D. F., Weinberg, R. A. & Greene, M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 41, 697–706 (1985).
Hudziak, R. M. et al. P185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell Biol. 9, 1165–1172 (1989).
Sarup, J. C. et al. Characterization of an anti-p185her2 monoclonal antibody that stimulates receptor function and inhibits tumor cell growth. Growth Regul. 1, 72–82 (1991).
Shepard, H. M. et al. Monoclonal antibody therapy of human cancer: taking the her2 protooncogene to the clinic. J. Clin. Immunol. 11, 117–127 (1991).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Naruse, I., Fukumoto, H., Saijo, N. & Nishio, K. Enhanced anti-tumor effect of trastuzumab in combination with cisplatin. Jpn. J. Cancer Res. 93, 574–581 (2002).
Carter, P. et al. Humanization of an anti-p185her2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 12, 49–55 (2001).
Baselga, J. et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14, 737–744 (1996).
Esteva, F. J. et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20, 1800–1808 (2002).
Pegram, M. D. et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 16, 2659–2671 (1998).
Pegram, M. D. et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J. Natl Cancer Inst. 96, 759–769 (2004).
Pegram, M. D. et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J. Natl Cancer Inst. 96, 739–749 (2004).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Smith, I. et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369, 29–36 (2007).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Xia, W. et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21, 6255–6263 (2002).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Gomez, H. L. et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol. 26, 2999–3005 (2008).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Esteva, F. J. & Hortobagyi, G. N. Gaining ground on breast cancer. Sci. Am. 298, 58–65 (2008).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Dybdal, N. et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res. Treat. 93, 3–11 (2005).
Gong, Y. et al. Chromogenic in situ hybridization is a reliable method for detecting HER2 gene status in breast cancer: a multicenter study using conventional scoring criteria and the new ASCO/CAP recommendations. Am. J. Clin. Pathol. 131, 490–497 (2009).
Paik, S. et al. Real-world performance of HER2 testing--National Surgical Adjuvant Breast and Bowel Project experience. J. Natl Cancer Inst. 94, 852–854 (2002).
Wolff, A. C. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 25, 118–145 (2007).
Esteva, F. J. et al. CD40 signaling predicts response to preoperative trastuzumab and concomitant paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide in HER-2-overexpressing breast cancer. Breast Cancer Res. 9, R87 (2007).
Untch, M. et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann. Oncol. 19, 1090–1096 (2008).
Seidman, A. D. et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J. Clin. Oncol. 26, 1642–1649 (2008).
Kaufman, P. A. et al. CALGB 150002: Correlation of HER2 and chromosome 17 copy number with trastuzumab (T) efficacy in CALGB 9840, paclitaxel with or without T in HER2+ and HER2- metastatic breast cancer [abstract]. J. Clin. Oncol. 25, a1009 (2007).
Paik, S., Kim, C. & Wolmark, N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N. Engl. J. Med. 358, 1409–1411 (2008).
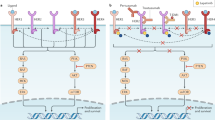
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Spivak-Kroizman, T. et al. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J. Biol. Chem. 267, 8056–8063 (1992).
Ferguson, K. M. et al. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell 11, 507–517 (2003).
Garrett, T. P. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505 (2003).
Cai, Z. et al. Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene 27, 3870–3874 (2008).
Scott, G. K. et al. p185HER2 signal transduction in breast cancer cells. J. Biol. Chem. 266, 14300–14305 (1991).
Nagata, Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6, 117–127 (2004).
Nahta, R., Yuan, L. X., Du, Y. & Esteva, F. J. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol. Cancer Ther. 6, 667–674 (2007).
Shi, Y. et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn. Mol. Pathol. 18, 11–21 (2009).
Molina, M. A. et al. NH2-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin. Cancer Res. 8, 347–353 (2002).
Esteva, F. J. et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 7, 436–443 (2005).
Saez, R. et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin. Cancer Res. 12, 424–431 (2006).
Scaltriti, M. et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl Cancer Inst. 99, 628–638 (2007).
Xia, W., Liu, L. H., Ho, P. & Spector, N. L. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene 23, 646–653 (2004).
Xia, W. et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24, 6213–6221 (2005).
Finak, G. et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 (2008).
Gong, Y. et al. Determination of estrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 8, 203–211 (2007).
Symmans, W. F. et al. Total RNA yield and microarray gene expression profiles from fine-needle aspiration biopsy and core-needle biopsy samples of breast carcinoma. Cancer 97, 2960–2971 (2003).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Thor, A. D. et al. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): a study of incidence and correlation with outcome in breast cancer. J. Clin. Oncol. 18, 3230–3239 (2000).
DiGiovanna, M. P. et al. Influence of activation state of ErbB-2 (HER-2) on response to adjuvant cyclophosphamide, doxorubicin, and fluorouracil for stage II, node-positive breast cancer: study 8541 from the Cancer and Leukemia Group B. J. Clin. Oncol. 26, 2364–2372 (2008).
Frogne, T., Laenkholm, A. V., Lyng, M. B., Henriksen, K. L. & Lykkesfeldt, A. E. Determination of HER2 phosphorylation at tyrosine 1221/1222 improves prediction of poor survival for breast cancer patients with hormone receptor-positive tumors. Breast Cancer Res. 11, R11 (2009).
Johnston, S. et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J. Clin. Oncol. 26, 1066–1072 (2008).
Meric-Bernstam, F. & Gonzalez-Angulo, A. M. Targeting the mTOR signaling network for cancer therapy. J. Clin. Oncol. 27, 2278–2287 (2009).
Berns, K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12, 395–402 (2007).
Yakes, F. M. et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 62, 4132–4141 (2002).
Eichhorn, P. J. et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 68, 9221–9230 (2008).
Stemke-Hale, K. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 68, 6084–6091 (2008).
Steck, P. A. et al. Identification of a candidate tumor suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Lu, C. H. et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin. Cancer Res. 13, 5883–5888 (2007).
Xia, W. et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homolog deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 67, 1170–1175 (2007).
Kim, C. et al. Trastuzumab sensitivity of breast cancer with coamplification of HER2 and C-MYC suggests proapoptotic function of dysregulated c-MYC in-vivo. Breast Cancer Res. Treat. 88 (Suppl. 1), S6a (2005).
Perez, E. A. et al. c-MYC amplification and correlation with patient outcome in early stage HER2+ breast cancer from the NCCTG adjuvant intergroup trial N9831 [abstract]. Breast Cancer Res. Treat. (Suppl.), a56 (2008).
Harris, L. N. et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin. Cancer Res. 13, 1198–1207 (2007).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Nahta, R. & Esteva, F. J. In vitro effects of trastuzumab and vinorelbine in trastuzumab-resistant breast cancer cells. Cancer Chemother. Pharmacol. 53, 186–190 (2004).
Burstein, H. J. et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J. Clin. Oncol. 21, 2889–2895 (2003).
Pirker, R. et al. Characterization of immunotoxins active against ovarian cancer cell lines. J. Clin. Invest. 76, 1261–1267 (1985).
Vogel, C. L. et al. A phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody-drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): Final results [abstract]. J. Clin. Oncol. 27 (Suppl.), a1017 (2009).
Adams, C. W. et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol. Immunother. 55, 717–727 (2006).
Agus, D. B. et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2, 127–137 (2002).
Nahta, R., Hung, M. C. & Esteva, F. J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 64, 2343–2346 (2004).
Lee-Hoeflich, S. T. et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 68, 5878–5887 (2008).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. (in press).
Baselga, J. et al. Objective response rate in a phase II multicenter trial of pertuzumab (P), a HER2 dimerization inhibiting monoclonal antibody, in combination with trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) which has progressed during treatment with T [abstract]. J. Clin. Oncol. 25 (Suppl. 18), a1004 (2007).
Oda, K. et al. PIK3CA cooperates with other phosphatidylinositol 3'-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 68, 8127–8136 (2008).
Junttila, T. T. et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15, 429–440 (2009).
Folkes, A. J. et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3, 2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 51, 5522–5532 (2008).
O'Reilly, K. E. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 (2006).
Lu, Y., Zi, X., Zhao, Y., Mascarenhas, D. & Pollak, M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (herceptin). J. Natl Cancer Inst. 93, 1852–1857 (2001).
Munster, P. N., Basso, A., Solit, D., Norton, L. & Rosen, N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule- dependent manner. Clin. Cancer Res. 7, 2228–2236 (2001).
Modi, S. et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J. Clin. Oncol. 25, 5410–5417 (2007).
Swaby, R. et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: A phase I/II study [abstract]. J. Clin. Oncol. 27 (Suppl.), a1004 (2009).
Tripathy, D. et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J. Clin. Oncol. 22, 1063–1070 (2004).
von Minckwitz, G. et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J. Clin. Oncol. 27, 1999–2006 (2009).
O'Shaughnessy, J. A. et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [abstract]. J. Clin. Oncol. 26, a101 (2008).
Mittendorf, E. A. et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 15, 7381–7388 (2009).
Xia, W. et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 66, 1640–1647 (2006).
Xia, W. et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc. Natl Acad. Sci. USA 103, 7795–7800 (2006).
Le, X. F. et al. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J. Biol. Chem. 278, 23441–23450 (2003).
Chu, I., Blackwell, K., Chen, S. & Slingerland, J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 65, 18–25 (2005).
Mohsin, S. K. et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J. Clin. Oncol. 23, 2460–2468 (2005).
Molina, M. A. et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 61, 4744–4749 (2001).
Izumi, Y., Xu, L., di Tomaso, E., Fukumura, D. & Jain, R. K. Tumor biology: herceptin acts as an anti-angiogenic cocktail. Nature 416, 279–280 (2002).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Lin, N. U. et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Kaufman, B. et al. Lapatinib monotherapy in patients with HER2-overexpressing relapsed or refractory inflammatory breast cancer: final results and survival of the expanded HER2+ cohort in EGF103009, a phase II study. Lancet Oncol. 10, 581–588 (2009).
Burstein, H. J. et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann. Oncol. 19, 1068–1074 (2008).
Cameron, D. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112, 533–543 (2008).
Acknowledgements
Supported in part by a Breast Cancer SPORE grant (NCI P50 CA116199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Francisco J. Esteva declares he receives research support from Genentech, GlaxoSmithKline, Monogram Biosciences, Myriad Genetics and Novartis. The funds are managed by the University of Texas M. D. Anderson Cancer Center. Gabriel N. Hortobagyi declares he is a consultant for Bristol-Myers Squibb and Novartis and receives research support from Novartis. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Esteva, F., Yu, D., Hung, MC. et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol 7, 98–107 (2010). https://doi.org/10.1038/nrclinonc.2009.216
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.216
This article is cited by
-
Identification of tumor biomarkers for pathological complete response to neoadjuvant treatment in locally advanced breast cancer
Breast Cancer Research and Treatment (2022)
-
Combination effect of lapatinib with foretinib in HER2 and MET co-activated experimental esophageal adenocarcinoma
Scientific Reports (2019)
-
Nuclear ErbB-2: a Novel Therapeutic Target in ErbB-2-Positive Breast Cancer?
Hormones and Cancer (2019)
-
MiR-16 mediates trastuzumab and lapatinib response in ErbB-2-positive breast and gastric cancer via its novel targets CCNJ and FUBP1
Oncogene (2016)
-
Ultra-deep tyrosine phosphoproteomics enabled by a phosphotyrosine superbinder
Nature Chemical Biology (2016)