Abstract
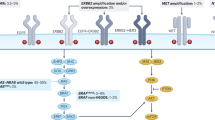
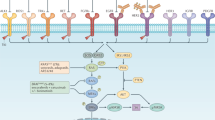
EGFR regulates cancer-cell proliferation, apoptosis and tumor-induced neoangiogenesis, and has been validated as a relevant therapeutic target in several human cancers, including metastatic colorectal cancer (mCRC). The anti-EGFR monoclonal antibodies cetuximab and panitumumab are available for the treatment of patients with mCRC. Although EGFR is expressed in approximately 85% of patients with mCRC, the clinical efficacy of treatment with anti-EGFR antibodies is limited to a subset of patients. A series of potential biomarkers that could be useful in predicting response to EGFR inhibitors has been investigated. In patients with mCRC, activating mutations within KRAS can predict resistance to anti-EGFR monoclonal antibodies. Activating mutations in KRAS, which could result in EGFR-independent intracellular signal transduction activation, are found in approximately 35–40% of patients with mCRC. These mutations are almost exclusively detected in codons 12 and 13 of exon 2. KRAS mutations have been significantly associated with lack of response to cetuximab or panitumumab therapy in patients with mCRC, which suggests that EGFR-independent, constitutive activation of the RAS signaling pathway could impair response to anti-EGFR drugs. We summarize the experimental and clinical evidence supporting the use of KRAS testing for the optimal selection of patients with mCRC to be treated with anti-EGFR monoclonal antibodies.
Key Points
-
Cetuximab and panitumumab are effective as single agents, and cetuximab is effective in combination with irinotecan, in heavily pretreated patients with chemorefractory metastatic colorectal cancer (mCRC)
-
Cetuximab in combination with standard chemotherapy is also active as first-line therapy in untreated patients with mCRC; however, efficacy of anti-EGFR monoclonal antibodies in mCRC is limited to a subgroup of patients
-
Several potential biomarkers for the optimal selection of patients with mCRC who are candidates for treatment with anti-EGFR drugs have been evaluated
-
Activating mutations in KRAS have been strongly correlated with lack of efficacy of anti-EGFR drugs in mCRC, whereas patients with wild-type KRAS respond to cetuximab or panitumumab monotherapy
-
Combination of cetuximab with standard 5-fluorouracil-based chemotherapy doublets is a valid therapeutic option for the first-line treatment of patients with wild-type KRAS mCRC
-
Determination of KRAS status should be mandatory in all patients with mCRC to help select the most appropriate combinations and/or sequences of molecular targeted drugs and cytotoxic agents
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Eng. J. Med. 358, 1160–1174 (2008).
Shepard, H. M., Brdlik, C. M. & Schreiber, H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J. Clin. Invest. 118, 3574–3581 (2008).
Normanno, N., Bianco, C., De Luca, A., Maiello, M. R. & Salomon, D. S. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr. Relat. Cancer 10, 1–21 (2003).
Malumbres, M. & Barbacid, M. RAS oncogenes: the first 30 years. Nat. Rev. Cancer 3, 459–465 (2003).
Koera, K. et al. K-ras is essential for the development of the mouse embryo. Oncogene 15, 1151–1159 (1997).
Johnson, L. et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 11, 2468–2481 (1997).
Haigis, K. M. et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat. Genet. 40, 600–608 (2008).
Schafer, W. R. et al. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science 249, 1133–1139 (1990).
Schubbert, S., Shannon, K. & Bollag, G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer 7, 295–308 (2007).
Rosen, N. Molecular biology of gastrointestinal cancers. In Cancer—Principles and Practice of Oncology 5th edn (Eds DeVita, V. T. J., Hellman, S. & Rosenberg, S. A.) 971–979 (Lippincott Williams & Wilkins, 1997).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Bos, J. L. et al. Prevalence of ras gene mutations in human colorectal cancers. Nature 327, 293–297 (1987).
Samowitz, W. S. et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 65, 6063–6069 (2005).
Ogino, S. et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 58, 90–96 (2009).
Takayama, T. et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology 121, 599–611 (2001).
Pretlow, T. P. & Pretlow, T. G. Mutant KRAS in aberrant crypt foci (ACF): initiation of colorectal cancer? Biochim. Biophys. Acta 1756, 83–96 (2005).
Santini, D. et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist 13, 1270–1275 (2008).
Artale, S. et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J. Clin. Oncol. 26, 4217–4219 (2008).
Forbes, S. et al. Cosmic 2005. Br. J. Cancer 94, 318–322 (2006).
Bos, J. L. ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 (1989).
Andreyev, H. J. et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br. J. Cancer 85, 692–696 (2001).
Benvenuti, S. et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 67, 2643–2648 (2007).
Di Nicolantonio, F. et al. Wild-type BRAF is required for response to panitumimab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 26, 5705–5712 (2008).
Andreyev, H. J., Norman, A. R., Cunningham, D., Oates, J. R. & Clarke, P. A. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J. Natl Cancer Inst. 90, 675–684 (1998).
Samowitz, W. S. et al. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol. Biomarkers Prev. 9, 1193–1197 (2000).
Westra, J. L. et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J. Clin. Oncol. 23, 5635–5643 (2005).
Barault, L. et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int. J. Cancer 122, 2255–2259 (2008).
Belly, R. T. et al. Detection of mutated K12-ras in histologically negative lymph nodes as an indicator of poor prognosis in stage II colorectal cancer. Clin. Colorectal Cancer 1, 110–116 (2001).
Moroni, M. et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 6, 279–286 (2005).
Lièvre, A. et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 66, 3992–3995 (2006).
Frattini, M. et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br. J. Cancer 9, 1139–1145 (2007).
Di Fiore, F. et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br. J. Cancer 96, 1166–1169 (2007).
Khambata-Ford, S. et al. Expression of epregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 25, 3230–3237 (2007).
De Roock, W. et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann. Oncol. 19, 508–515 (2008).
Lièvre, A. et al. KRAS mutation as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 26, 374–379 (2008).
Bibeau, F. et al. Impact of FcγRIIa–FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J. Clin. Oncol. 27, 1122–1129 (2009).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Karapetis, C. S. et al. KRAS mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008).
Cervantes, A. et al. Correlation of KRAS status (wild type [wt] vs. mutant [mt]) with efficacy to first-line cetuximab in a study of cetuximab single agent followed by cetuximab + FOLFIRI in patients (pts) with metastatic colorectal cancer (mCRC) [abstract]. ASCO Meeting Abstracts 26, 4129 (2008).
Folprecht, G. et al. Cetuximab plus FOLFOX or cetuximab plus FOLFIRI as neoadjuvant treatment of nonresectable colorectal liver metastases: A randomized multicenter study (CELIM study) [abstract]. Gastrointestinal Cancer Symposium 2009: 296 (2009).
Bokemeyer, C. et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 27, 663–671 (2008).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Allegra, C. J. et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene muations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 27, 2091–2096 (2009).
van Krieken, J. H. et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch. 454, 233–235 (2008).
Jimeno, A., Messersmith, W. A., Hirsch, F. R., Franklin, W. A. & Eckhardt, S. G. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J. Clin. Oncol. 27, 1130–1136 (2009).
Ogino, S. et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J. Mol. Diagn. 3, 413–421 (2005).
Juan, T. et al. A comparability study of 4 commercial KRAS tests [abstract]. AACR Meeting Abstracts 2008: 1811 (2008).
Linardou, H. et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 10, 962–972 (2008).
Cappuzzo, F. et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann. Oncol. 19, 717–723 (2008).
Personeni, N. et al. Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin. Cancer Res. 14, 5869–5876 (2008).
De Roock, W., Lambrechts, D. & Tejpar, S. K-ras mutations and cetuximab in colorectal cancer. N. Engl. J. Med. 360, 834 (2009).
Perrone, F. et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann. Oncol. 20, 84–90 (2009).
Sartore-Bianchi, A. et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 69, 1851–1857 (2009).
Loupakis, F. et al. Evaluation of PTEN expression in colorectal cancer (CRC) metastases (mets) and in primary tumors as predictors of activity of cetuximab plus irinotecan treatment [abstract]. ASCO Meeting Abstracts 26, 4003 (2008).
Morgillo, F., Bareschino, M. A., Bianco, R., Tortora, G. & Ciardiello, F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation 75, 788–799 (2007).
Jorissen, R. N. et al. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53 (2003).
Yordy, J. S. & Muise-Helmericks, R. C. Signal transduction and the Ets family of transcription factors. Oncogene 19, 6503–6513 (2000).
Malliri, A. et al. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature 417, 867–871 (2002).
Wolthuis, R. M. & Bos, J. L. Ras caught in another affair: the exchange factors for Ral. Curr. Opin. Genet. Dev. 9, 112–117 (1999).
Kelley, G. G., Reks, S. E., Ondrako, J. M. & Smrcka, A. V. Phospholipase C(epsilon): a novel Ras effector. EMBO J. 20, 743–754 (2001).
Song, C. et al. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J. Biol. Chem. 276, 2752–2757 (2001).
Acknowledgements
Research projects in the laboratories of F. Ciardiello and N. Normanno are partly supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy. S. Tejpar and E. Van Cutsem are Senior Clinical Investigators of the Fund for Scientific Research, Flanders. Their work is partly supported by a grant of the Belgian Foundation against Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E. Van Cutsem declares he receives grant/research support from Amgen and Merck Serono. F. Ciardiello declares he receives grant/research support from Merck Serono. S. Tejpar declares she receives grant/research support from Merck Serono and Pfizer. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Normanno, N., Tejpar, S., Morgillo, F. et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 6, 519–527 (2009). https://doi.org/10.1038/nrclinonc.2009.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.111
This article is cited by
-
Novel heavily fucosylated glycans as a promising therapeutic target in colorectal cancer
Journal of Translational Medicine (2023)
-
YB-1 activating cascades as potential targets in KRAS-mutated tumors
Strahlentherapie und Onkologie (2023)
-
Depleting receptor tyrosine kinases EGFR and HER2 overcomes resistance to EGFR inhibitors in colorectal cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
CD46 protects the bladder cancer cells from cetuximab-mediated cytotoxicity
Scientific Reports (2022)
-
Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers
Journal of Biomedical Science (2021)