Key Points
-
Mendelian randomization (MR) is a powerful tool that utilizes genetic information to inform about the likely causal relevance of an exposure to an outcome
-
When performed rigorously, MR findings should be free from reverse causality bias, and only minimally affected by confounding
-
The number of MR studies has been increasing in the past decade, providing important new insights into disease aetiology
-
However, as MR studies become more common, and as increasingly complex gene-to-exposure and exposure-to-outcome relationships are investigated, reliable conduct and interpretation of MR analyses can be challenging
-
Potential solutions to aid the conduct and interpretation of MR studies can be derived, for example, through use of emerging statistical approaches to investigate potential genetic pleiotropy that can distort the findings
Abstract
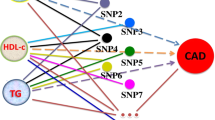
Mendelian randomization (MR) is a burgeoning field that involves the use of genetic variants to assess causal relationships between exposures and outcomes. MR studies can be straightforward; for example, genetic variants within or near the encoding locus that is associated with protein concentrations can help to assess their causal role in disease. However, a more complex relationship between the genetic variants and an exposure can make findings from MR more difficult to interpret. In this Review, we describe some of these challenges in interpreting MR analyses, including those from studies using genetic variants to assess causality of multiple traits (such as branched-chain amino acids and risk of diabetes mellitus); studies describing pleiotropic variants (for example, C-reactive protein and its contribution to coronary heart disease); and those investigating variants that disrupt normal function of an exposure (for example, HDL cholesterol or IL-6 and coronary heart disease). Furthermore, MR studies on variants that encode enzymes responsible for the metabolism of an exposure (such as alcohol) are discussed, in addition to those assessing the effects of variants on time-dependent exposures (extracellular superoxide dismutase), cumulative exposures (LDL cholesterol), and overlapping exposures (triglycerides and non-HDL cholesterol). We elaborate on the molecular features of each relationship, and provide explanations for the likely causal associations. In doing so, we hope to contribute towards more reliable evaluations of MR findings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fewell, Z., Davey Smith, G. & Sterne, J. A. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am. J. Epidemiol. 166, 646–655 (2007).
Davey Smith, G. et al. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 4, e352 (2007).
Harrison, R. K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 15, 817–818 (2016).
Fordyce, C. B. et al. Cardiovascular drug development: is it dead or just hibernating? J. Am. Coll. Cardiol. 65, 1567–1582 (2015).
Wang, Q. et al. Effects of hormonal contraception on systemic metabolism: cross-sectional and longitudinal evidence. Int. J. Epidemiol. 45, 1445–1457 (2016).
Corrao, G., Rubbiati, L., Bagnardi, V., Zambon, A. & Poikolainen, K. Alcohol and coronary heart disease: a meta-analysis. Addiction 95, 1505–1523 (2000).
Marmot, M. & Brunner, E. Alcohol and cardiovascular disease: the status of the U shaped curve. BMJ 303, 565–568 (1991).
Kloner, R. A. & Rezkalla, S. H. To drink or not to drink? That is the question. Circulation 116, 1306–1317 (2007).
Mukamal, K. J. & Rimm, E. B. Alcohol's effects on the risk for coronary heart disease. Alcohol Res. Health 25, 255–261 (2001).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98 (2014).
Davey Smith, G. & Ebrahim, S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22 (2003).
Swerdlow, D. I. et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int. J. Epidemiol. 45, 1600–1616 (2016).
Evans, D. M. & Davey Smith, G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu. Rev. Genomics Hum. Genet. 16, 327–350 (2015).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Ference, B. A. et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J. Am. Coll. Cardiol. 60, 2631–2639 (2012).
Holmes, M. V. et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 36, 539–550 (2015).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380, 572–580 (2012).
White, J. et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 1, 692–699 (2016).
Cholesterol Treatment Trialists' Collaborators et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Silverman, M. G. et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 316, 1289–1297 (2016).
C Reactive Protein Coronary Heart Disease Genetics Collaboration et al. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 342, d548 (2011).
Zacho, J. et al. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 359, 1897–1908 (2008).
Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349, g4164 (2014).
Hagg, S. et al. Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int. J. Epidemiol. 44, 578–586 (2015).
Nordestgaard, B. G. et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med. 9, e1001212 (2012).
Emdin, C. A. et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 317, 626–634 (2017).
Dale, C. et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes and type 2 diabetes: a Mendelian randomization analysis. Circulation http://dx.doi.org/10.1161/CIRCULATIONAHA.116.026560
Look AHEAD Research Group et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369, 145–154 (2013).
Wurtz, P. et al. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J. Am. Coll. Cardiol. 67, 1200–1210 (2016).
Swerdlow, D. I. et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 385, 351–361 (2014).
Holmes, M. V. et al. Secretory phospholipase A2-IIA and cardiovascular disease: a Mendelian randomization study. J. Am. Coll. Cardiol. 62, 1966–1976 (2013).
Nicholls, S. J. et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 311, 252–262 (2014).
Polfus, L. M., Gibbs, R. A. & Boerwinkle, E. Coronary heart disease and genetic variants with low phospholipase A2 activity. N. Engl. J. Med. 372, 295–296 (2015).
STABILITY Investigators et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 370, 1702–1711 (2014).
Millwood, I. Y. et al. Lipoprotein-associated phospholipase A2 loss-of-function variant and risk of vascular diseases in 90,000 Chinese adults. J. Am. Coll. Cardiol. 67, 230–231 (2016).
Millwood, I. Y. et al. A phenome-wide association study of a lipoprotein-associated phospholipase A2 loss-of-function variant in 90 000 Chinese adults. Int. J. Epidemiol. 45, 1588–1599 (2016).
Talmud, P. J. & Holmes, M. V. Deciphering the causal role of sPLA2s and Lp-PLA2 in coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 35, 2281–2289 (2015).
Ference, B. A., Majeed, F., Penumetcha, R., Flack, J. M. & Brook, R. D. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial Mendelian randomization study. J. Am. Coll. Cardiol. 65, 1552–1561 (2015).
Schmidt, A. F. et al. PCSK9 genetic variants and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 5, 97–105 (2017).
Ference, B. A. et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375, 2144–2153 (2016).
Lotta, L. A. et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA 316, 1383–1391 (2016).
Guasch-Ferre, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
Soininen, P., Kangas, A. J., Wurtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 8, 192–206 (2015).
Wurtz, P. et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655 (2013).
Wurtz, P. et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 35, 1749–1756 (2012).
Wurtz, P. et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 61, 1372–1380 (2012).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Lotta, L. A. et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 13, e1002179 (2016).
Elliott, P. et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 302, 37–48 (2009).
Evans, D. M. et al. Mining the human phenome using allelic scores that index biological intermediates. PLoS Genet. 9, e1003919 (2013).
Prins, B. P. et al. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium Mendelian randomization study. PLoS Med. 13, e1001976 (2016).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Zanoni, P. et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 351, 1166–1171 (2016).
Ledford, H. 'Good' cholesterol mutation linked to heart disease. Nature http://www.nature.com/news/good-cholesterol-mutation-linked-to-heart-disease-1.19543?WT.mc_id=FBK_NA_1603_NEWSCHOLESTEROLMUTATION_PORTFOLIO (2016).
Harb, J. Why having too much 'good' cholesterol can actually be BAD for you. Daily Mail http://www.dailymail.co.uk/health/article-3384473/Why-having-good-cholesterol-actually-BAD-you.html (2016).
Tall, A. R. An overview of reverse cholesterol transport. Eur. Heart J. 19 (Suppl. A), A31–A35 (1998).
Rosenson, R. S. et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012).
Zhang, Y. et al. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 115, 2870–2874 (2005).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099 (2012).
Eli Lilly and Company. Lilly to discontinue development of evacetrapib for high-risk atherosclerotic cardiovascular disease. Lilly https://investor.lilly.com/releasedetail.cfm?ReleaseID=936130 (2015).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371, 2383–2393 (2014).
Anastasius, M. et al. Cholesterol efflux capacity: an introduction for clinicians. Am. Heart J. 180, 54–63 (2016).
Rosenson, R. S. et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 13, 48–60 (2016).
Kingwell, B. A., Chapman, M. J., Kontush, A. & Miller, N. E. HDL-targeted therapies: progress, failures and future. Nat. Rev. Drug Discov. 13, 445–464 (2014).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Lissilaa, R. et al. Although IL-6 trans-signaling is sufficient to drive local immune responses, classical IL-6 signaling is obligate for the induction of T cell-mediated autoimmunity. J. Immunol. 185, 5512–5521 (2010).
IL6R Genetics Consortium Emerging Risk Factors Collaboration et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 379, 1205–1213 (2012).
Interleukin-6 Receptor Mendelian Randomisation Analysis Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 379, 1214–1224 (2012).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Rafiq, S. et al. A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects. Genes Immun. 8, 552–559 (2007).
Mullberg, J. et al. The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site. J. Immunol. 152, 4958–4968 (1994).
Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 8, 1237–1247 (2012).
Kleveland, O. et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 37, 2406–2413 (2016).
Richmond, R. C., Hemani, G., Tilling, K., Davey Smith, G. & Relton, C. L. Challenges and novel approaches for investigating molecular mediation. Hum. Mol. Genet. 25, R149–R156 (2016).
Secretan, B. et al. A review of human carcinogens — part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 10, 1033–1034 (2009).
Cho, Y. et al. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci. Rep. 5, 18422 (2015).
Brooks, P. J., Enoch, M. A., Goldman, D., Li, T. K. & Yokoyama, A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 6, e50 (2009).
Peng, G. S. & Yin, S. J. Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum. Genomics 3, 121–127 (2009).
Marmot, M. G. et al. Alcohol and blood pressure: the INTERSALT study. BMJ 308, 1263–1267 (1994).
Chen, L., Davey Smith, G., Harbord, R. M. & Lewis, S. J. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 5, e52 (2008).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163 (2008).
Davey Smith, G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. 6, 27–43 (2011).
Tabara, Y. et al. Mendelian randomization analysis in three Japanese populations supports a causal role of alcohol consumption in lowering low-density lipid cholesterol levels and particle numbers. Atherosclerosis 254, 242–248 (2016).
Lewis, S. J. & Davey Smith, G. Alcohol, ALDH2, and esophageal cancer: a meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol. Biomarkers Prev. 14, 1967–1971 (2005).
Zuccolo, L. & Holmes, M. V. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int. J. Epidemiol. http://dx.doi.org/10.1093/ije/dyw327 (2016).
Mokry, L. E. et al. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med. 12, e1001866 (2015).
Cabre, P. Migration and multiple sclerosis: the French West Indies experience. J. Neurol. Sci. 262, 117–121 (2007).
Dean, G. & Elian, M. Age at immigration to England of Asian and Caribbean immigrants and the risk of developing multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 63, 565–568 (1997).
Elian, M., Nightingale, S. & Dean, G. Multiple sclerosis among United Kingdom-born children of immigrants from the Indian subcontinent, Africa and the West Indies. J. Neurol. Neurosurg. Psychiatry 53, 906–911 (1990).
Dean, G. & Kurtzke, J. F. On the risk of multiple sclerosis according to age at immigration to South Africa. Br. Med. J. 3, 725–729 (1971).
Navab, M. et al. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler. Thromb. Vasc. Biol. 16, 831–842 (1996).
Myung, S. K. et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 346, f10 (2013).
Vivekananthan, D. P., Penn, M. S., Sapp, S. K., Hsu, A. & Topol, E. J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361, 2017–2023 (2003).
Powers, K. M., Oberley, L. W. & Domann, F. E. in Oxidants in Biology: A Question of Balance (eds Valacchi, G. & Davis, P. A.) 183–201 (Springer, 2008).
Jung, O. et al. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ. Res. 93, 622–629 (2003).
Gongora, M. C. et al. Role of extracellular superoxide dismutase in hypertension. Hypertension 48, 473–481 (2006).
Juul, K. et al. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation 109, 59–65 (2004).
Siedlinski, M., van Diemen, C. C., Postma, D. S., Vonk, J. M. & Boezen, H. M. Superoxide dismutases, lung function and bronchial responsiveness in a general population. Eur. Respir. J. 33, 986–992 (2009).
Young, R. P. et al. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax 61, 394–399 (2006).
Juul, K., Tybjaerg-Hansen, A., Marklund, S., Lange, P. & Nordestgaard, B. G. Genetically increased antioxidative protection and decreased chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 173, 858–864 (2006).
Kobylecki, C. J., Afzal, S. & Nordestgaard, B. G. Does SOD3 R213G homozygosity influence morbidity, mortality, and lung function in the general population? Antioxid. Redox Signal. 24, 884–891 (2016).
Hartney, J. M. et al. A common polymorphism in extracellular superoxide dismutase affects cardiopulmonary disease risk by altering protein distribution. Circ. Cardiovasc. Genet. 7, 659–666 (2014).
Kobylecki, C. J., Afzal, S., Davey Smith, G. & Nordestgaard, B. G. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am. J. Clin. Nutr. 101, 1135–1143 (2015).
Cook, N. R. et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch. Intern. Med. 167, 1610–1618 (2007).
Cholesterol Treatment Trialists' Collaborators et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380, 581–590 (2012).
Davey Smith, G. & Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42 (2004).
McGill, H. C. Jr et al. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 72, 1307S–1315S (2000).
Strong, J. P., Malcom, G. T., Newman, W. P. III & Oalmann, M. C. Early lesions of atherosclerosis in childhood and youth: natural history and risk factors. J. Am. Coll. Nutr. 11 (Suppl.), 51S–54S (1992).
Rosengren, A. et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur. Heart J. 27, 789–795 (2006).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260 (2015).
Aschard, H., Vilhjalmsson, B. J., Joshi, A. D., Price, A. L. & Kraft, P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am. J. Hum. Genet. 96, 329–339 (2015).
Richiardi, L., Bellocco, R. & Zugna, D. Mediation analysis in epidemiology: methods, interpretation and bias. Int. J. Epidemiol. 42, 1511–1519 (2013).
Helgadottir, A. et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat. Genet. 48, 634–639 (2016).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Niemi, J. et al. Estimation of VLDL, IDL, LDL, HDL2, apoA-I, and apoB from the Friedewald inputs — apoB and IDL, but not LDL, are associated with mortality in type 1 diabetes. Ann. Med. 41, 451–461 (2009).
Shah, T. et al. Gene-centric analysis identifies variants associated with interleukin-6 levels and shared pathways with other inflammation markers. Circ. Cardiovasc. Genet. 6, 163–170 (2013).
Fall, T. et al. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 10, e1001474 (2013).
Burgess, S., Dudbridge, F. & Thompson, S. G. Re: “Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects”. Am. J. Epidemiol. 181, 290–291 (2015).
Hartwig, F. P., Davey Smith, G. & Bowden, J. Robust inference in two-sample Mendelian randomisation via the zero modal pleiotropy assumption. Int. J. Epidemiol. (in press).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802 (2017).
Ala-Korpela, M. & Davey Smith, G. Metabolic profiling-multitude of technologies with great research potential, but (when) will translation emerge? Int. J. Epidemiol. 45, 1311–1318 (2016).
Mundra, P. A., Shaw, J. E. & Meikle, P. J. Lipidomic analyses in epidemiology. Int. J. Epidemiol. 45, 1329–1338 (2016).
Ganz, P. et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA 315, 2532–2541 (2016).
Hartwig, F. P., Davies, N. M., Hemani, G. & Davey Smith, G. Two-sample Mendelian randomisation: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 6, 1717–1726 (2016).
Khera, A. V. & Kathiresan, S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat. Rev. Genet. http://dx.doi.org/10.1038/nrg.2016.160 (2017).
Hemani, G. et al. MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. Preprint at bioRxiv http://dx.doi.org/10.1101/078972 (2016).
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017).
Staley, J. R. et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics 32, 3207–3209 (2016).
Davey Smith, G., Paternoster, L. & Relton, C. When will Mendelian randomization become relevant for clinical practice and public health? JAMA 317, 589–591 (2017).
Gibson, G. Rare and common variants: twenty arguments. Nat. Rev. Genet. 13, 135–145 (2012).
Bush, W. S. & Moore, J. H. Chapter 11: genome-wide association studies. PLoS Comput. Biol. 8, e1002822 (2012).
Global Lipids Genetics Consortium et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Merino, J. et al. Genetically driven hyperglycemia increases risk of coronary artery disease separately from type 2 diabetes. Diabetes Care 40, 687–693 (2017).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45, 1961–1974 (2016).
Wurtz, P. et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 11, e1001765 (2014).
Emerging Risk Factors Collaboration et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Casas, J. P. et al. PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European Ancestry. Circulation 121, 2284–2293 (2010).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Myocardial Infarction Genetics Consortium Investigators et al. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 371, 2072–2082 (2014).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Cohen, J. C., Boerwinkle, E., Mosley, T. H. Jr & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Clarke, R. et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361, 2518–2528 (2009).
Dewey, F. E. et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 374, 1123–1133 (2016).
Ridker, P. M., Pradhan, A., MacFadyen, J. G., Libby, P. & Glynn, R. J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 380, 565–571 (2012).
Sattar, N. et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375, 735–742 (2010).
Piepoli, M. F. et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
Yun, K. E. et al. Alcohol and coronary artery calcification: an investigation using alcohol flushing as an instrumental variable. Int. J. Epidemiol. http://dx.doi.org/10.1093/ije/dyw237 (2017).
Acknowledgements
M.A.-K. is supported by Biocenter Oulu and Sigrid Juselius Foundation, Finland. M.A.-K. and G.D.S. work in a unit that receives funding from the University of Bristol and UK Medical Research Council (MC_UU_12013/1). M.V.H. works in a unit that receives funds from the University of Oxford and the UK Medical Research Council. These funding bodies did not have a role in the study design, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, and made substantial contributions to discussion of content, writing, reviewing, and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Randomized, controlled trials (RCTs)
-
An interventional study in which individuals are randomized to an 'exposed' arm (for example, an active drug) or to a comparator control arm (for example, placebo) to test the efficacy of an intervention on an outcome (such as disease risk). Such trials are considered the gold standard for asserting causal relationships of an exposure to disease risk.
- Mendelian randomization
-
A genetic epidemiological approach that aims to quantify the causal relationship between an exposure and an outcome, by using the properties of the genome (randomized owing to Mendel's second law, and invariant) to minimize the issues of confounding and reverse causality that can undermine traditional observational epidemiology.
- Vertical pleiotropy
-
The association of a genetic marker (for example, a single nucleotide polymorphism [SNP] in isolation, or a genetic instrument consisting of multiple SNPs) with more than one phenotype on the same biological pathway.
- Horizontal pleiotropy
-
The association of a genetic marker with more than one phenotype on discrete biological pathways.
- Genome-wide association study (GWAS)
-
The hypothesis-free investigation of hundreds of thousands to millions of genetic variants (typically single nucleotide polymorphisms) for their association with a phenotype to characterize the underlying genetic architecture of the phenotype.
- Linkage disequilibrium
-
The nonrandom assortment of genetic variants, meaning that when linkage disequilibrium between a pair of variants is high (for example, as measured by a r2 value of >0.80), a single nucleotide polymorphism (SNP) can be used as a 'proxy' for another SNP in the absence of this second SNP being directly genotyped.
- Unbalanced horizontal pleiotropy
-
When horizontal pleiotropy is such that alternative pathways from the genetic marker to disease can lead to distortion of the association of the exposure under investigation. Unbalanced horizontal pleiotrophy is a violation of the exclusion restriction assumption of an instrumental variable.
- Instrumental variable
-
A variable used as a proxy for an exposure of interest that is not associated with confounders and only associates with an outcome through the exposure of interest
- Friedewald equation
-
The estimation of LDL-cholesterol levels in the absence of its direct measurement, from total cholesterol, high-density lipoprotein cholesterol, and triglycerides
- Two-sample Mendelian randomization
-
The single nucleotide polymorphism (SNP)-to-exposure estimate is obtained from a separate dataset to that of the SNP-to-outcome estimate.
Rights and permissions
About this article
Cite this article
Holmes, M., Ala-Korpela, M. & Smith, G. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol 14, 577–590 (2017). https://doi.org/10.1038/nrcardio.2017.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.78
This article is cited by
-
Mendelian randomization analysis reveals causal relationship between obstetric-related diseases and COVID-19
Virology Journal (2024)
-
Serum electrolyte concentrations and risk of atrial fibrillation: an observational and mendelian randomization study
BMC Genomics (2024)
-
Peripheral blood indicators and COVID-19: an observational and bidirectional Mendelian randomization study
BMC Medical Genomics (2024)
-
Lifestyle factors, glycemic traits, and lipoprotein traits and risk of liver cancer: a Mendelian randomization analysis
Scientific Reports (2024)
-
A two-sample Mendelian randomization study explores metabolic profiling of different glycemic traits
Communications Biology (2024)