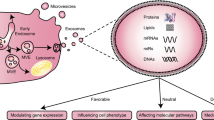
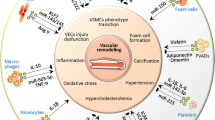
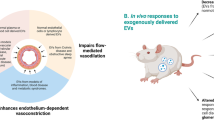
Key Points
-
Extracellular vesicles are released in biological fluids both under basal conditions and in pathological settings
-
Extracellular vesicles act as vectors of biological information by transferring their protein, lipid, and nucleic acid content to target cells
-
Circulating levels of extracellular vesicles of different cellular origin are increased in cardiovascular diseases, including myocardial infarction; these extracellular vesicles could serve as diagnostic and prognostic biomarkers
-
Extracellular vesicles might contribute to atherosclerosis development and progression by promoting initial lesion formation, intravascular calcifications, unstable plaque progression, and thrombus formation after rupture
-
Extracellular vesicles released by stem cells (progenitor or mesenchymal stem cells) have potential therapeutic benefits on cardiac function after myocardial infarction
Abstract
Membrane vesicles released in the extracellular space are composed of a lipid bilayer enclosing soluble cytosolic material and nuclear components. Extracellular vesicles include apoptotic bodies, exosomes, and microvesicles (also known previously as microparticles). Originating from different subcellular compartments, the role of extracellular vesicles as regulators of transfer of biological information, acting locally and remotely, is now acknowledged. Circulating vesicles released from platelets, erythrocytes, leukocytes, and endothelial cells contain potential valuable biological information for biomarker discovery in primary and secondary prevention of coronary artery disease. Extracellular vesicles also accumulate in human atherosclerotic plaques, where they affect major biological pathways, including inflammation, proliferation, thrombosis, calcification, and vasoactive responses. Extracellular vesicles also recapitulate the beneficial effect of stem cells to treat cardiac consequences of acute myocardial infarction, and now emerge as an attractive alternative to cell therapy, opening new avenues to vectorize biological information to target tissues. Although interest in microvesicles in the cardiovascular field emerged about 2 decades ago, that for extracellular vesicles, in particular exosomes, started to unfold a decade ago, opening new research and therapeutic avenues. This Review summarizes current knowledge on the role of extracellular vesicles in coronary artery disease, and their emerging potential as biomarkers and therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 13, 269–288 (1967).
van der Pol, E., Boing, A. N., Gool, E. L. & Nieuwland, R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J. Thromb. Haemost. 14, 48–56 (2016).
Yanez-Mo, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell.Vesicles 4, 27066 (2015).
Morel, O., Jesel, L., Freyssinet, J. M. & Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 31, 15–26 (2011).
Sapet, C. et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108, 1868–1876 (2006).
Vion, A. C. et al. Shear stress regulates endothelial microparticle release. Circ. Res. 112, 1323–1333 (2013).
Shet, A. S. et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 102, 2678–2683 (2003).
Connor, D. E., Exner, T., Ma, D. D. & Joseph, J. E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 103, 1044–1052 (2010).
Arraud, N. et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12, 614–627 (2014).
Osteikoetxea, X. et al. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS ONE 10, e0121184 (2015).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977 (2016).
Varga, Z. et al. Towards traceable size determination of extracellular vesicles. J. Extracell. Vesicles 3, 23298 (2014).
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
Boing, A. N. et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 3, 23430 (2014).
Lacroix, R. et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J. Thromb. Haemost. 10, 437–446 (2012).
van der Pol, E., Coumans, F., Varga, Z., Krumrey, M. & Nieuwland, R. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 11 (Suppl. 1), 36–45 (2013).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 (2014).
Augustine, D. et al. Dynamic release and clearance of circulating microparticles during cardiac stress. Circ. Res. 114, 109–113 (2014).
Dasgupta, S. K. et al. Lactadherin and clearance of platelet-derived microvesicles. Blood 113, 1332–1339 (2009).
Dasgupta, S. K., Le, A., Chavakis, T., Rumbaut, R. E. & Thiagarajan, P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 125, 1664–1672 (2012).
Ratajczak, M. Z. & Ratajczak, J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin. Transl. Med. 5, 7 (2016).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
Nordin, J. Z. et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 11, 879–883 (2015).
Chevillet, J. R. et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl Acad. Sci. USA 111, 14888–14893 (2014).
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D. & Remaley, A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 13, 423–433 (2011).
Arroyo, J. D. et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl Acad. Sci. USA 108, 5003–5008 (2011).
Castoldi, M., Kordes, C., Sawitza, I. & Haussinger, D. Isolation and characterization of vesicular and non-vesicular microRNAs circulating in sera of partially hepatectomized rats. Sci. Rep. 6, 31869 (2016).
Wiklander, O. P. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015).
Willekens, F. L. et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 105, 2141–2145 (2005).
Al Faraj, A. et al. Endothelial cell-derived microparticles loaded with iron oxide nanoparticles: feasibility of MR imaging monitoring in mice. Radiology 263, 169–178 (2012).
Happonen, K. E. et al. The Gas6–Axl protein interaction mediates endothelial uptake of platelet microparticles. J. Biol. Chem. 291, 10586–10601 (2016).
Svensson, K. J. et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17713–17724 (2013).
Tian, T. et al. Dynamics of exosome internalization and trafficking. J. Cell. Physiol. 228, 1487–1495 (2013).
Fitzner, D. et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 (2011).
Tian, T. et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 289, 22258–22267 (2014).
del Conde, I. et al. Effect of P-selectin on phosphatidylserine exposure and surface-dependent thrombin generation on monocytes. Arterioscler. Thromb. Vasc. Biol. 25, 1065–1070 (2005).
Parolini, I. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009).
Feng, D. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687 (2010).
Soares, A. R. et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 5, 13243 (2015).
Heusermann, W. et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 213, 173–184 (2016).
Bobryshev, Y. V., Killingsworth, M. C. & Orekhov, A. N. Increased shedding of microvesicles from intimal smooth muscle cells in athero-prone areas of the human aorta: implications for understanding of the predisease stage. Pathobiology 80, 24–31 (2013).
Leroyer, A. S. et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 49, 772–777 (2007).
Mayr, M. et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ. Cardiovasc. Genet. 2, 379–388 (2009).
Amabile, N., Rautou, P. E., Tedgui, A. & Boulanger, C. M. Microparticles: key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 36, 907–916 (2010).
Perrotta, I. & Aquila, S. Exosomes in human atherosclerosis: an ultrastructural analysis study. Ultrastruct. Pathol. 40, 101–106 (2016).
Arteaga, R. B. et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am. J. Cardiol. 98, 70–74 (2006).
Amabile, N. et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 35, 2972–2979 (2014).
Gordon, C. et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am. J. Respir. Crit. Care Med. 184, 224–232 (2011).
Heiss, C. et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 51, 1760–1771 (2008).
Li, C. J., Liu, Y., Chen, Y., Yu, D., Williams, K. J. & Liu, M. L. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am. J. Pathol. 182, 1552–1562 (2013).
Ferreira, A. C. et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 110, 3599–3603 (2004).
Koga, H. et al. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur. Heart J. 27, 817–823 (2006).
Diamant, M. et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 106, 2442–2447 (2002).
Nomura, S. et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis 116, 235–240 (1995).
Koga, H. et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 45, 1622–1630 (2005).
Sabatier, F. et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 51, 2840–2845 (2002).
Preston, R. A. et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41, 211–217 (2003).
Nomura, S., Shouzu, A., Omoto, S., Nishikawa, M. & Iwasaka, T. Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin. Appl. Thromb. Hemost. 10, 133–141 (2004).
Chironi, G. et al. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler. Thromb. Vasc. Biol. 26, 2775–2780 (2006).
Suades, R., Padro, T., Vilahur, G. & Badimon, L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 108, 1208–1219 (2012).
Suades, R., Padro, T., Alonso, R., Mata, P. & Badimon, L. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb. Haemost. 114, 1310–1321 (2015).
Barry, O. P., Pratico, D., Savani, R. C. & FitzGerald, G. A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Invest. 102, 136–144 (1998).
Mesri, M. & Altieri, D. C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 161, 4382–4387 (1998).
Mesri, M. & Altieri, D. C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 274, 23111–23118 (1999).
Nomura, S. et al. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 158, 277–287 (2001).
Njock, M. S. et al. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 125, 3202–3212 (2015).
Agouni, A. et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 173, 1210–1219 (2008).
Amabile, N. et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 16, 3381–3388 (2005).
Boulanger, C. M. et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104, 2649–2652 (2001).
Brodsky, S. V., Zhang, F., Nasjletti, A. & Goligorsky, M. S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol. 286, H1910–H1915 (2004).
Densmore, J. C. et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 26, 464–471 (2006).
Jansen, F. et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 98, 94–106 (2013).
Dean, W. L., Lee, M. J., Cummins, T. D., Schultz, D. J. & Powell, D. W. Proteomic and functional characterisation of platelet microparticle size classes. Thromb. Haemost. 102, 711–718 (2009).
Edrissi, H., Schock, S. C., Hakim, A. M. & Thompson, C. S. Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro. Brain Res. 1634, 83–93 (2016).
Marcos-Ramiro, B. et al. Microparticles in multiple sclerosis and clinically isolated syndrome: effect on endothelial barrier function. BMC Neurosci. 15, 110 (2014).
Huber, J. et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 22, 101–107 (2002).
Mause, S. F., von Hundelshausen, P., Zernecke, A., Koenen, R. R. & Weber, C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 (2005).
Rautou, P. E. et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 108, 335–343 (2011).
Fink, K. et al. Selenium prevents microparticle-induced endothelial inflammation in patients after cardiopulmonary resuscitation. Crit. Care 19, 58 (2015).
Curtis, A. M. et al. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J. Thromb. Haemost. 7, 701–709 (2009).
Soleti, R. et al. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 30, 580–588 (2009).
Jansen, F. et al. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J. Cell. Mol. Med. 19, 2202–2214 (2015).
Forlow, S. B., McEver, R. P. & Nollert, M. U. Leukocyte–leukocyte interactions mediated by platelet microparticles under flow. Blood 95, 1317–1323 (2000).
Zu, L. et al. Endothelial microparticles after antihypertensive and lipid-lowering therapy inhibit the adhesion of monocytes to endothelial cells. Int. J. Cardiol. 202, 756–759 (2016).
Keyel, P. A., Tkacheva, O. A., Larregina, A. T. & Salter, R. D. Coordinate stimulation of macrophages by microparticles and TLR ligands induces foam cell formation. J. Immunol. 189, 4621–4629 (2012).
Zakharova, L., Svetlova, M. & Fomina, A. F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 212, 174–181 (2007).
Yahagi, K. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 13, 79–98 (2016).
Boing, A. N., Hau, C. M., Sturk, A. & Nieuwland, R. Platelet microparticles contain active caspase 3. Platelets 19, 96–103 (2008).
Distler, J. H. et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 10, 731–741 (2005).
Huber, L. C. et al. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis 12, 363–374 (2007).
Abid Hussein, M. N. et al. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J. Thromb. Haemost. 3, 888–896 (2005).
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Sarkar, A., Mitra, S., Mehta, S., Raices, R. & Wewers, M. D. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS ONE 4, e7140 (2009).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Llorente-Cortes, V., Otero-Vinas, M., Camino-Lopez, S., Llampayas, O. & Badimon, L. Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation 110, 452–459 (2004).
Pakala, R. Serotonin and thromboxane A2 stimulate platelet-derived microparticle-induced smooth muscle cell proliferation. Cardiovasc. Radiat. Med. 5, 20–26 (2004).
Weber, A., Koppen, H. O. & Schror, K. Platelet-derived microparticles stimulate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb. Res. 98, 461–466 (2000).
Brousseau, C., Morissette, G., Fortin, J. P., Marceau, F. & Petitclerc, E. Tumor cells expressing tissue factor influence the migration of smooth muscle cells in a catalytic activity-dependent way. Can. J. Physiol. Pharmacol. 87, 694–701 (2009).
Shan, Z. et al. An endocrine genetic signal between blood cells and vascular smooth muscle cells: role of microRNA-223 in smooth muscle function and atherogenesis. J. Am. Coll. Cardiol. 65, 2526–2537 (2015).
Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
Zhou, J. et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ. Res. 113, 40–51 (2013).
Jansen, F. et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 3, e001249 (2014).
Sinning, J. M. et al. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 32, 2034–2041 (2011).
Werner, N., Wassmann, S., Ahlers, P., Kosiol, S. & Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 26, 112–116 (2006).
Bernal-Mizrachi, L. et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 145, 962–970 (2003).
Nozaki, T. et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll. Cardiol. 54, 601–608 (2009).
Lacroix, R. et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J. Thromb. Haemost. 11, 1190–1193 (2013).
Yuana, Y. et al. Handling and storage of human body fluids for analysis of extracellular vesicles. J. Extracell. Vesicles 4, 29260 (2015).
Otsuka, F., Sakakura, K., Yahagi, K., Joner, M. & Virmani, R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler. Thromb. Vasc. Biol. 34, 724–736 (2014).
Kelly-Arnold, A. et al. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc. Natl Acad. Sci. USA 110, 10741–10746 (2013).
Sangiorgi, G. et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 31, 126–133 (1998).
Burke, P. A. et al. Pathophysiology of calcium deposition in coronary arteries. Herz 26, 239–244 (2001).
Huang, H. et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103, 1051–1056 (2001).
Nicholls, S. J. et al. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J. Am. Coll. Cardiol. 49, 263–270 (2007).
Vengrenyuk, Y. et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl Acad. Sci. USA 103, 14678–14683 (2006).
Criqui, M. H. et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 311, 271–278 (2014).
Martin, S. S. et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 129, 77–86 (2014).
Hsu, J. J., Lim, J., Tintut, Y. & Demer, L. L. Cell-matrix mechanics and pattern formation in inflammatory cardiovascular calcification. Heart 102, 1710–1715 (2016).
Buendia, P. et al. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 29, 173–181 (2015).
Kapustin, A. N. et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 116, 1312–1323 (2015).
Kapustin, A. N. et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 109, e1–e12 (2011).
New, S. E. P. et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 113, 72–77 (2013).
Reynolds, J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15, 2857–2867 (2004).
Hutcheson, J. D. et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 15, 335–343 (2016).
Goettsch, C. et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Invest. 126, 1323–1336 (2016).
New, S. E. P. & Aikawa, E. Role of extracellular vesicles in de novo mineralization: an additional novel mechanism of cardiovascular calcification. Arterioscler. Thromb. Vasc. Biol. 33, 1753–1758 (2013).
Mallat, Z. et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 99, 348–353 (1999).
Min, P. K. et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 227, 323–328 (2013).
Porto, I. et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 33, 2928–2938 (2012).
Morel, O., Toti, F., Morel, N. & Freyssinet, J. M. Microparticles in endothelial cell and vascular homeostasis: are they really noxious? Haematologica 94, 313–317 (2009).
Falati, S. et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 197, 1585–1598 (2003).
Merten, M., Pakala, R., Thiagarajan, P. & Benedict, C. R. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation 99, 2577–2582 (1999).
Srikanthan, S., Li, W., Silverstein, R. L. & McIntyre, T. M. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J. Thromb. Haemost. 12, 1906–1917 (2014).
Michel, J.-B., Virmani, R., Arbustini, E. & Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 32, 1977–1985 (2011).
Camus, S. M. et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood 120, 5050–5058 (2012).
Camus, S. M. et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 125, 3805–3814 (2015).
Leroyer, A. S. et al. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J. Am. Coll. Cardiol. 52, 1302–1311 (2008).
Lacroix, R. et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 110, 2432–2439 (2007).
Marcos-Ramiro, B., Garcia-Weber, D. & Millan, J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb. Haemost. 112, 1088–1102 (2014).
Falk, E., Nakano, M., Bentzon, J. F., Finn, A. V. & Virmani, R. Update on acute coronary syndromes: the pathologists' view. Eur. Heart J. 34, 719–728 (2013).
Janiszewski, M. et al. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit. Care Med. 32, 818–825 (2004).
Lozito, T. P. & Tuan, R. S. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. J. Cell. Physiol. 227, 534–549 (2012).
Martinez de Lizarrondo, S. et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 32, 1477–1487 (2012).
Taraboletti, G. et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160, 673–680 (2002).
Gasser, O. et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 285, 243–257 (2003).
Canault, M. et al. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 171, 1713–1723 (2007).
Folkesson, M. et al. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb. Haemost. 114, 1165–1174 (2015).
Aharon, A., Tamari, T. & Brenner, B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 100, 878–885 (2008).
Mitra, S., Wewers, M. D. & Sarkar, A. Mononuclear phagocyte-derived microparticulate caspase-1 induces pulmonary vascular endothelial cell injury. PLoS ONE 10, e0145607 (2015).
Schock, S. C. et al. Microparticles generated during chronic cerebral ischemia deliver proapoptotic signals to cultured endothelial cells. Biochem. Biophys. Res. Commun. 450, 912–917 (2014).
Narula, J. et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J. Am. Coll. Cardiol. 61, 1041–1051 (2013).
Otsuka, F., Joner, M., Prati, F., Virmani, R. & Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 11, 379–389 (2014).
Katopodis, J. N. et al. Platelet microparticles and calcium homeostasis in acute coronary ischemias. Am. J. Hematol. 54, 95–101 (1997).
Mallat, Z. et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 101, 841–843 (2000).
Montoro-Garcia, S. et al. Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 227, 313–322 (2013).
Morel, O. et al. Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thromb. Haemost. 91, 345–353 (2004).
Zielinska, M. et al. Circulating endothelial microparticles in patients with acute myocardial infarction. Kardiol. Pol. 62, 531–542 (2005).
van der Zee, P. M. et al. P-Selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin. Chem. 52, 657–664 (2006).
Giannopoulos, G. et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 176, 145–150 (2014).
Biasucci, L. M. et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 76, 2174–2182 (2012).
Stepien, E. et al. Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch. Med. Res. 43, 31–35 (2012).
Sarlon-Bartoli, G. et al. Plasmatic level of leukocyte-derived microparticles is associated with unstable plaque in asymptomatic patients with high-grade carotid stenosis. J. Am. Coll. Cardiol. 62, 1436–1441 (2013).
Abbas, M. et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cells ageing and thrombogenicity: role of the Ang II/AT1 receptor/NADPH oxidase-mediated activation of MAPKs and PI3-kinase pathways. Circulation 135, 280–296 (2016).
Chiva-Blanch, G. et al. CD3+/CD45+ and SMA-alpha+ circulating microparticles are increased in individuals at high cardiovascular risk who will develop a major cardiovascular event. Int. J. Cardiol. 208, 147–149 (2016).
Tsiantoulas, D. et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 56, 440–448 (2015).
Emanueli, C. et al. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS ONE 11, e0154274 (2016).
Bi, S. et al. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int. J. Clin. Exp. Med. 8, 4275–4280 (2015).
Matsumoto, S. et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 113, 322–326 (2013).
de Hoog, V. C. et al. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2, 53–60 (2013).
Brill, A., Dashevsky, O., Rivo, J., Gozal, Y. & Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 67, 30–38 (2005).
Vicencio, J. M. et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 65, 1525–1536 (2015).
Lai, C. P. & Breakefield, X. O. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 3, 228 (2012).
Lai, R. C., Chen, T. S. & Lim, S. K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen. Med. 6, 481–492 (2011).
Timmers, L. et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 1, 129–137 (2007).
Timmers, L. et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 6, 206–214 (2011).
Arslan, F. et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312 (2013).
Gnecchi, M. et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 11, 367–368 (2005).
Gnecchi, M. et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 20, 661–669 (2006).
Kervadec, A. et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transplant. 35, 795–807 (2016).
Lai, R. C. et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4, 214–222 (2010).
Chen, L. et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 431, 566–571 (2013).
Ibrahim, A. G., Cheng, K. & Marban, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehw240 (2016).
Vader, P., Mol, E. A., Pasterkamp, G. & Schiffelers, R. M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 106, 148–156 (2016).
Ferguson, S. W. & Nguyen, J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Control. Release 228, 179–190 (2016).
Zernecke, A. et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2, ra81 (2009).
Jansen, F. et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 128, 2026–2038 (2013).
Mackie, A. R. et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 111, 312–321 (2012).
Agouni, A. et al. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 21, 2735–2741 (2007).
Benameur, T., Soleti, R., Porro, C., Andriantsitohaina, R. & Martinez, M. C. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS ONE 5, e12688 (2010).
Goto, S. et al. Different effects of various anti-GPIIb-IIIa agents on shear-induced platelet activation and expression of procoagulant activity. J. Thromb. Haemost. 1, 2022–2030 (2003).
Shouzu, A. et al. Effect of ticlopidine on monocyte-derived microparticles and activated platelet markers in diabetes mellitus. Clin. Appl. Thromb. Hemost 10, 167–173 (2004).
Morel, O. et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J. Thromb. Haemost. 2, 1118–1126 (2004).
Kagawa, H., Nomura, S., Nagahama, M., Ozaki, Y. & Fukuhara, S. Effect of ticlopidine on platelet-derived microparticles in patients with connective tissue diseases. Haemostasis 29, 255–261 (1999).
Nomura, S., Shouzu, A., Omoto, S., Nishikawa, M. & Iwasaka, T. Benidipine improves oxidized LDL-dependent monocyte and endothelial dysfunction in hypertensive patients with type 2 diabetes mellitus. J. Hum. Hypertens. 19, 551–557 (2005).
Nomura, S. et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets 20, 16–22 (2009).
Nomura, S. et al. Effects of pitavastatin on monocyte chemoattractant protein-1 in hyperlipidemic patients. Blood Coagul. Fibrinolysis 20, 440–447 (2009).
Tramontano, A. F. et al. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho–kinase pathway. Biochem. Biophys. Res. Commun. 320, 34–38 (2004).
Diamant, M. et al. Simvastatin-induced endothelial cell detachment and microparticle release are prenylation dependent. Thromb. Haemost. 100, 489–497 (2008).
Huang, B. et al. Effect of 40 mg versus 10 mg of atorvastatin on oxidized low-density lipoprotein, high-sensitivity C-reactive protein, circulating endothelial-derived microparticles, and endothelial progenitor cells in patients with ischemic cardiomyopathy. Clin. Cardiol. 35, 125–130 (2012).
Suades, R., Padro, T., Alonso, R., Mata, P. & Badimon, L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb. Haemost. 110, 366–377 (2013).
Almquist, T., Mobarrez, F., Jacobson, S. H., Wallen, H. & Hjemdahl, P. Effects of lipid-lowering treatment on circulating microparticles in patients with diabetes mellitus and chronic kidney disease. Nephrol. Dial. Transplant. 31, 944–952 (2016).
Camargo, L. M. et al. Effects of simvastatin/ezetimibe on microparticles, endothelial progenitor cells and platelet aggregation in subjects with coronary heart disease under antiplatelet therapy. Braz. J. Med. Biol. Res. 47, 432–437 (2014).
Mobarrez, F. et al. Release of endothelial microparticles in vivo during atorvastatin treatment; a randomized double-blind placebo-controlled study. Thromb. Res. 129, 95–97 (2012).
Gerrits, A. J., Koekman, C. A., Yildirim, C., Nieuwland, R. & Akkerman, J. W. Insulin inhibits tissue factor expression in monocytes. J. Thromb. Haemost. 7, 198–205 (2009).
Acknowledgements
The authors were supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Paris Descartes University, the Fondation de France 2012–00029497 (C.M.B.), ITMO-PMN-2016 (X.L.), Agence Nationale de la Recherche ANR-2016-Extrema (X.L.), ANR-2011-META-MiRA (C.M.B.), and ANR-Emergence-2012-MicroSpy (P.-E.R.).
Author information
Authors and Affiliations
Contributions
C.M.B., X.L., and N.A. researched data for the article and wrote the manuscript. C.M.B. and P.-E.R. reviewed and edited the manuscript before submission. All authors provided substantial contribution to the discussion of content.
Corresponding author
Ethics declarations
Competing interests
C.M.B. and P.-E.R. have filed patents on the detection and biomarker use of microvesicles. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Boulanger, C., Loyer, X., Rautou, PE. et al. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 14, 259–272 (2017). https://doi.org/10.1038/nrcardio.2017.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.7
This article is cited by
-
Research progress in the management of vascular disease with cannabidiol: a review
Journal of Cardiothoracic Surgery (2024)
-
Extracellular vesicles and their effect on vascular haemodynamics: a systematic review
Hypertension Research (2024)
-
Lipoprotein apheresis affects the concentration of extracellular vesicles in patients with elevated lipoprotein (a)
Scientific Reports (2024)
-
Extracellular microvesicles: biologic properties, biogenesis, and applications in leukemia
Molecular and Cellular Biochemistry (2024)
-
Development of stem cell therapy for atherosclerosis
Molecular and Cellular Biochemistry (2024)