Key Points
-
The role of elevated blood triglycerides as an independent risk factor for cardiovascular disease (CVD) has been debated for more than half a century, but without resolution
-
One of the reasons for this uncertainty is that hypertriglyceridaemia is often associated with decreased HDL-cholesterol levels and an increased number of atherogenic small dense LDL particles
-
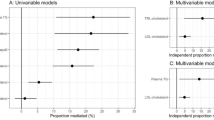
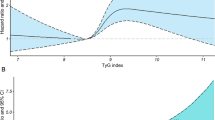
Large observational, epidemiological, genetic, and Mendelian randomization studies support the hypothesis that elevated blood triglyceride levels, either fasting or nonfasting, are independently associated with an increased risk of CVD
-
Therapeutic targeting of hypertriglyceridaemia might provide further benefit in reducing CVD and events, in addition to that achieved with LDL-cholesterol lowering
-
Hypertriglyceridaemia is currently treated with lifestyle interventions and with fibrates, which can be combined with high doses of omega-3 fatty acids, but CVD outcome studies have produced inconsistent results
-
Some novel drugs, such as pemafibrate and volanesorsen, are being tested, but clinical data are preliminary
Abstract
An elevated serum level of LDL cholesterol is a well-known risk factor for cardiovascular disease (CVD), but the role of elevated triglyceride levels is debated. Controversies regarding hypertriglyceridaemia as an independent risk factor for CVD have occurred partly because elevated triglyceride levels are often a component of atherogenic dyslipidaemia — they are associated with decreased levels of HDL cholesterol and increased levels of small dense LDL particles, which are highly atherogenic. Findings from several large studies indicate that elevated levels of triglycerides (either fasting or nonfasting) or, more specifically, triglyceride-rich lipoproteins and their remnants, are independently associated with increased risk of CVD. Possible mechanisms for this association include excessive free fatty acid release, production of proinflammatory cytokines, coagulation factors, and impairment of fibrinolysis. Therapeutic targeting of hypertriglyceridaemia could, therefore, reduce CVD and cardiovascular events, beyond the reduction achieved by LDL-cholesterol lowering. Elevated triglyceride levels are reduced with lifestyle interventions and fibrates, which can be combined with omega-3 fatty acids. Some new drugs are on the horizon, such as volanesorsen (which targets apolipoprotein C-III), pemafibrate, and others. However, CVD outcome studies with triglyceride-lowering agents have produced inconsistent results, meaning that no convincing evidence is available that lowering triglycerides by any approach can reduce mortality.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reiner, Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 10, 453–464 (2013).
Graham, I. et al. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr. Cardiol. Rep. 14, 709–720 (2012).
Reiner, Ž. Management of patients with familial hypercholesterolaemia. Nat. Rev. Cardiol. 12, 565–575 (2015).
De Backer, G. et al. Prevalence and management of familial hypercholesterolaemia in coronary patients: an analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis 241, 169–175 (2015).
Carroll, M. D. et al. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA 308, 1545–1554 (2012).
Nordestgaard, B. G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 118, 547–563 (2016).
Wang, H. & Eckel, R. H. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297, E271–E288 (2009).
Surendran, R. P. et al. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J. Intern. Med. 272, 185–196 (2012).
Goldberg, I. J. et al. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J. Biol. Chem. 265, 4266–4272 (1990).
Mehta, N. et al. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arterioscler. Thromb. Vasc. Biol. 34, 1057–1063 (2014).
Hegele, R. A. et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2, 655–666 (2014).
Goldstein, J. L. et al. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J. Clin. Invest. 52, 1544–1568 (1973).
Veerkamp, M. J. et al. Diagnosis of familial combined hyperlipidemia based on lipid phenotype expression in 32 families: results of a 5-year follow-up study. Arterioscler. Thromb. Vasc. Biol. 22, 274–282 (2002).
Chait, A., Subramanian, S. & Brunzell, J. D. Genetic disorders of triglyceride metabolism. Endotext https://www.ncbi.nlm.nih.gov/books/NBK326743/ (2015).
Johansen, C. T. et al. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 31, 1916–1926 (2011).
Norata, G. D. et al. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol. Sci. 36, 675–687 (2015).
Rashid, S. et al. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation 130, 431–441 (2014).
Jacobson, T. A. et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 — executive summary. J. Clin. Lipidol. 8, 473–488 (2014).
Klempfner, R. et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ. Cardiovasc. Qual. Outcomes 9, 100–108 (2016).
Schwartz, G. G. et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 65, 2267–2275 (2015).
Carey, V. J. et al. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am. J. Cardiol. 106, 757–763 (2010).
Johansen, C. T. et al. Mutation skew in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 42, 684–687 (2010).
Bansal, S. et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298, 309–316 (2007).
Nordestgaard, B. G. et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298, 299–308 (2007).
Nordestgaard, B. G. et al. European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 37, 1944–1958 (2016).
Hulley, S. B. et al. Epidemiology as a guide to clinical decisions. The association between triglyceride and coronary heart disease. N. Engl. J. Med. 302, 1383–1389 (1980).
Austin, M. A. et al. Genome-wide scan for quantitative trait loci influencing LDL size and plasma triglyceride in familial hypertriglyceridemia. J. Lipid Res. 44, 2161–2168 (2003).
Rosenson, R. S. et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 13, 48–60 (2016).
Catapano, A. L. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Atherosclerosis 253, 281–344 (2016).
Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members. An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia — full report. J. Clin. Lipidol. 8, 29–60 (2014).
Fruchart, J. C. et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidemic patients. A position paper by the Residual Risk Reduction Initiative (R3I). Diab. Vasc. Dis. Res. 4, 319–335 (2008).
Chapman, M. J. et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 332, 1345–1361 (2011).
Stenswold, I. et al. Non fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged Norwegian women. BMJ 307, 1318–1322 (1993).
Tuerdal, A. et al. Serum triglycerides as an independent risk factor for death from coronary heart disease in middle aged Norwegian men. Am. J. Epidemiol. 129, 458–465 (1989).
Stanvenow, L. & Kjellström, T. Influence of serum triglyceride levels on the risk for myocardial infarction in 12 510 middle aged males: interaction with serum cholesterol. Atherosclerosis 147, 243–247 (1999).
Jeppesen, J. et al. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 97, 1029–1036 (1998).
Hokanson, J. E. & Austin, M. A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 3, 213–219 (1996).
Sarwar, N. et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 115, 450–458 (2007).
Faergeman, O. et al. Plasma triglycerides and cardiovascular events in the Treating to New Targets and Incremental Decrease in End-Points through Aggressive Lipid Lowering trials of statins in patients with coronary artery disease. Am. J. Cardiol. 104, 459–463 (2009).
Criqui, M. H. et al. Plasma triglyceride level and mortality from coronary heart disease. N. Engl. J. Med. 328, 1220–1225 (1993).
Liu, J. et al. Impact of diabetes, high triglycerides and low HDL cholesterol on risk for ischemic cardiovascular disease varies by LDL cholesterol level: a 15-year follow-up of the Chinese Multi-provincial Cohort Study. Diabetes Res. Clin. Pract. 96, 217–224 (2012).
Nordestgaard, B. G. & Varbo, A. Triglycerides and cardiovascular disease. Lancet 384, 626–635 (2014).
Fruchart, J. C. et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am. J. Cardiol. 102, 1K–34K (2008).
Reiner, Ž. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients: a clinical update. Nutr. Metab. Cardiovasc. Dis. 23, 799–807 (2013).
Ferrari, R. et al. Current practice in identifying and treating cardiovascular risk, with focus on residual risk associated with atherogenic dyslipidaemia. Eur. Heart J. 18, C2–C12 (2016).
Kim, E. et al. Serum triglyceride levels and cardiovascular disease events in Koreans. Cardiology 131, 228–235 (2015).
Iso, H. et al. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis 237, 361–368 (2014).
Egeland, G. M. et al. Non-fasting triglycerides predict incident acute myocardial infarction among those with favourable HDL-cholesterol: Cohort Norway. Eur. J. Prev. Cardiol. 22, 872–881 (2015).
Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Sarwar, N. et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet 375, 1634–1639 (2010).
Puri, R. et al. Non-HDL cholesterol and triglycerides. Implications for coronary atheroma progression and clinical events. Arterioscler. Thromb. Vasc. Biol. 36, 2220–2228 (2016).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Thomsen, M. et al. Low nonfasting triglycerides and reduced all-cause mortality: a Mendelian randomization study. Clin. Chem. 60, 737–746 (2014).
Miller, M. et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 51, 724–730 (2008).
Holmes, M. V. et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 36, 539–550 (2015).
Dewey, F. E. et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 374, 1123–1133 (2016).
Stitziel, N. O. et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N. Engl. J. Med. 374, 1134–1144 (2016).
Khetarpal, S. A. & Rader, D. J. Triglyceride-rich lipoproteins and coronary artery disease risk. New insights from human genetics. Arterioscler. Thromb. Vasc. Biol. 35, 3–9 (2015).
Nordestgaard, B. G., Stender, S. & Kjeldsen, K. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 8, 421–428 (1988).
Rapp, J. H. et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 14, 1767–1774 (1994).
Goldstein, J. L. et al. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J. Biol. Chem. 255, 1839–1848 (1980).
Alaupovic, P. et al. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler. Thromb. Vasc. Biol. 17, 715–722 (1997).
Thorin, E. Vascular disease risk in patients with hypertriglyceridemia: endothelial progenitor cells, oxidative stress, accelerated senescence, and impaired vascular repair. Can. J. Cardiol. 27, 538–540 (2011).
Aung, H. H. et al. Induction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 33, 2088–2096 (2013).
Greene, D. J., Skeggs, J. W. & Morton, R. E. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI). J. Biol. Chem. 276, 4804–4811 (2001).
Skeggs, J. W. & Morton, R. E. LDL and HDL enriched in triglyceride promote abnormal cholesterol transport. J. Lipid. Res. 43, 1264–1274 (2002).
Rosenson, R. S. et al. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 64, 2525–2540 (2014).
Wang, Y. I. et al. Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS ONE 8, e78322 (2013).
Kajikawa, M. et al. Relationship between serum triglyceride levels and endothelial function in a large community-based study. Atherosclerosis 249, 70–75 (2016).
Wang, X. et al. Triglycerides are a predictive factor for arterial stiffness: a community-based 4.8-year prospective study. Lipids Health Dis. 15, 97 (2016).
Reiner, Ž. et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 32, 1769–1818 (2011).
Perk, J. et al. European guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 33, 1635–1717 (2012).
Goff, D. C. Jr et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49–S73 (2014).
Ray, K. K. et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur. Heart J. 35, 960–968 (2014).
Reiner, Ž., Sonicki, Z. & Tedeschi-Reiner, E. Physicians' perception, knowledge and awareness of cardiovascular risk factors and adherence to prevention guidelines: the PERCRO-DOC survey. Atherosclerosis 213, 598–603 (2010).
Reiner, Ž., Sonicki, Z. & Tedeschi-Reiner, E. Public perceptions of cardiovascular risk factors in Croatia: the PERCRO survey. Prev. Med. 51, 494–496 (2010).
Sacks, F. M. et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation 129, 999–1008 (2014).
Hermans, M. P. et al. Residual microvascular risk in type 2 diabetes in 2014: is it time for a re-think? A perspective from the Residual Risk Reduction Initiative (R3i). J. Diabetes Metab. 5, 8 (2014).
Reiner, Ž. et al. Treatment potential for dyslipidaemia management in patients with coronary heart disease across Europe: findings from the EUROASPIRE III survey. Atherosclerosis 231, 300–307 (2013).
Toth, P. P. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag. 12, 171–183 (2016).
Tenenbaum, A., Klempfner, R. & Fisman, E. Z. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc. Diabetol. 13, 159 (2014).
Borén, J. et al. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta 431, 131–142 (2014).
Reiner, Ž. Are elevated serum triglycerides really a risk factor for coronary artery disease? Cardiology 131, 225–227 (2015).
Carbajo, M. A. et al. Weight loss and improvement of lipid profiles in morbidly obese patients after laparoscopic one-anastomosis gastric bypass: 2-year follow-up. Surg. Endosc. 31, 416–421 (2016).
Dattilo, A. M. & Kris-Etherton, P. M. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am. J. Clin. Nutr. 56, 320–328 (1992).
Nordmann, A. J. et al. Effects of low-carbohydrate versus low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 166, 285–293 (2006).
Vanhees, L. et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR (part II). Eur. J. Prev. Cardiol. 19, 1005–1033 (2012).
Mann, J. Dietary carbohydrate: relationship to cardiovascular disease and disorders of carbohydrate metabolism. Eur. J. Clin. Nutr. 61, 100–111 (2007).
Stanhope, K. L. et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 119, 1322–1334 (2009).
Chun, S. et al. Sugar-sweetened carbonated beverage consumption and coronary artery calcification in asymptomatic men and women. Am. Heart J. 177, 17–24 (2016).
de Koning, L. et al. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 125, 1735–1741 (2012).
Stanhope, K. L. & Havel, P. J. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am. J. Clin. Nutr. 88, 1733–1737 (2008).
Karlson, B. W. et al. A VOYAGER meta-analysis of the impact of statin therapy on low-density lipoprotein cholesterol and triglyceride levels in patients with hypertriglyceridemia. Am. J. Cardiol. 117, 1444–1448 (2016).
Karalis, D. G. et al. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. Am. J. Cardiol. 100, 445–449 (2007).
Frick, M. H. et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 317, 1237–1245 (1987).
Keech, A. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861 (2005).
Robins, S. J. et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA 285, 1585–1591 (2001).
Jun, M. et al. Effects of fibrates on cardiovascular outcomes: a systemic review and meta-analysis. Lancet 375, 1875–1884 (2010).
DAIS Investigators. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357, 905–910 (2001).
Hiukka, A. et al. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J. Am. Coll. Cardiol. 52, 2190–2197 (2008).
The Bezafibrate Infarction Prevention (BIP) Study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 102, 21–27 (2000).
Aguiar, C. et al. A review of the evidence on reducing macrovascular risk in patients with atherogenic dyslipidaemia: a report from an expert consensus meeting on the role of fenofibrate-statin combination therapy. Atheroscler. Suppl. 19, 1–12 (2015).
ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
Ginsberg, H. N. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) Lipid trial: what we learn from subgroup analyses. Diabetes Care. 34 (Suppl. 2), S107–S108 (2011).
Sacks, F. M., Carey, V. J. & Fruchart, J. C. Combination lipid therapy in type 2 diabetes. N. Engl. J. Med. 363, 692–694 (2010).
Simic, I. & Reiner, Ž. Adverse effects of statins — myths and reality. Curr. Pharm. Des. 21, 1220–1226 (2015).
Holoshitz, N., Alsheikh-Ali, A. A. & Karas, R. H. Relative safety of gemfibrozil and fenofibrate in the absence of concomitant cerivastatin use. Am. J. Cardiol. 10, 95–97 (2008).
Kotwal, S. et al. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 5, 808–818 (2012).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00317707 (2012).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00135226 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01169259 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02104817 (2017).
Nelson, S. D. & Munger, M. A. Icosapent ethyl for treatment of elevated triglyceride levels. Ann. Pharmacother. 47, 1517–1523 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01492361 (2016).
Kastelein, J. J. et al. Icosabutate, a structurally engineered fatty acid, improves the cardiovascular risk profile in statin-treated patients with residual hypertriglyceridemia. Cardiology 135, 3–12 (2016).
Graham, M. J. et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res 112, 1479–1490 (2013).
Gaudet, D. et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 373, 438–447 (2015).
Yang, X. et al. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J. Lipid Res. 57, 706–713 (2016).
Pecin, I., Nedic, M. & Reiner, Ž. Volanesorsen (ISIS-APOCIII-LRx). Drug Future 41, 417–421 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02211209 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02300233 (2016).
Ishibashi, S. et al. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 249, 36–43 (2016).
Miller, M. et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011).
Hennuyer, N. et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis 249, 200–208 (2016).
The Business Journals. Landmark trial entitled “PROMINENT” to explore the prevention of heart disease in diabetic patients with high triglycerides and low HDL-C. The Business Journals http://www.bizjournals.com/prnewswire/press_releases/2016/01/12/CL94522 (2016).
Gaudet, D., Methot, J. & Kastelein, J. Gene therapy for lipoprotein lipase deficiency. Curr. Opin. Lipidol. 23, 310–320 (2012).
Meyers, C. D. et al. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 14, 8 (2015).
Kurano, M. et al. Genome-wide association study of serum lipids confirms previously reported associations as well as new associations of common SNPs within PCSK7 gene with triglyceride. J. Hum. Genet. 61, 427–433 (2016).
Schlein, C. et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab. 23, 441–453 (2016).
Wang, Y. et al. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J. Lipid Res. 56, 1296–1307 (2015).
Wierzbicki, A. S. & Viljoen, A. Anti-sense oligonucleotide therapies for the treatment of hyperlipidaemia. Expert Opin. Biol. Ther. 16, 1125–1134 (2016).
Makoveichuk, E. et al. Inactivation of LPL occurs on the surface of THP-1 macrophages where oligomers of ANGPTL4 are formed. Biochem. Biophys. Res. Commun. 425, 138–143 (2012).
Reiner, Ž. Combined therapy in the treatment of dyslipidemia. Fundam. Clin. Pharmacol. 24, 19–28 (2010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Reiner, Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol 14, 401–411 (2017). https://doi.org/10.1038/nrcardio.2017.31
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.31
This article is cited by
-
Dyslipidemia and associated factors among adult cardiac patients: a hospital-based comparative cross-sectional study
European Journal of Medical Research (2024)
-
Research progress in the management of vascular disease with cannabidiol: a review
Journal of Cardiothoracic Surgery (2024)
-
Elevated blood remnant cholesterol and triglycerides are causally related to the risks of cardiometabolic multimorbidity
Nature Communications (2024)
-
Elevated Kallistatin promotes the occurrence and progression of non-alcoholic fatty liver disease
Signal Transduction and Targeted Therapy (2024)
-
Effects of elevated remnant cholesterol on outcomes of acute ischemic stroke patients receiving mechanical thrombectomy
Journal of Thrombosis and Thrombolysis (2024)