Abstract
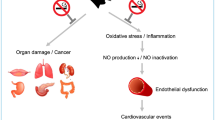
Cigarette smoke is an aerosol that contains >4,000 chemicals, including nicotine, carbon monoxide, acrolein, and oxidant compounds. Exposure to cigarette smoke induces multiple pathological effects in the endothelium, several of which are the result of oxidative stress initiated by reactive oxygen species, reactive nitrogen species, and other oxidant constituents of cigarette smoke. Cigarette-smoke exposure interferes adversely with the control of all stages of plaque formation and development and pathological thrombus formation. The reactive oxygen species in cigarette smoke contribute to oxidative stress, upregulation of inflammatory cytokines, and endothelial dysfunction, by reducing the bioavailability of nitric oxide. Plaque formation and the development of vulnerable plaques also result from exposure to cigarette smoke via the enhancement of inflammatory processes and the activation of matrix metalloproteases. Moreover, exposure to cigarette smoke results in platelet activation, stimulation of the coagulation cascade, and impairment of anticoagulative fibrinolysis. Many cigarette-smoke-mediated prothrombotic changes are quickly reversible upon smoking cessation. Public health efforts should urgently promote our understanding of current cigarette-smoke-induced cardiovascular pathology to encourage individuals to reduce their exposure to cigarette smoke and, therefore, the detrimental consequences of associated atherothrombotic disease.
Key Points
-
A large body of literature strongly suggests that cigarette smoke unfavourably influences all major stages of atherosclerosis as well as pathological atherothrombus formation
-
Cigarette smoke contains oxidant compounds that cause oxidative stress in the endothelium, leading to endothelial dysfunction and injury, initiation of the atherosclerotic process, and subsequent formation of atherosclerotic plaques
-
Cigarette smoke promotes the development of vulnerable plaques and plaque rupture by enhancing inflammation and activating matrix metalloproteinases
-
Cigarette smoke causes platelet activation, and promotes platelet aggregation and platelet adhesion to sites of endothelial injury
-
Exposure to cigarette smoke shifts the balance of haemostasis towards thrombus formation by enhancing blood clotting and, at the same time, reducing the fibrinolytic capacity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ezzati, M., Henley, S. J., Thun, M. J. & Lopez, A. D. Role of smoking in global and regional cardiovascular mortality. Circulation 112, 489–497 (2005).
Cornel, J. H. et al. Prior smoking status, clinical outcomes, and the comparison of ticagrelor with clopidogrel in acute coronary syndromes-Insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. Am. Heart J. 164, 334–342e1 (2012).
Burke, A. P. et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 336, 1276–1282 (1997).
Pope, C. A. III et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation 120, 941–948 (2009).
Smith, C. J. & Fischer, T. H. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis 158, 257–267 (2001).
Csordas, A., Wick, G., Laufer, G. & Bernhard, D. An evaluation of the clinical evidence on the role of inflammation and oxidative stress in smoking-mediated cardiovascular disease. Biomark. Insights 3, 127–139 (2008).
Pryor, W. A. & Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. NY Acad. Sci. 686, 12–27 (1993).
Lippi, G., Franchini, M. & Targher, G. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 8, 502–512 (2011).
Fuster, V., Moreno, P. R., Fayad, Z. A., Corti, R. & Badimon, J. J. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 46, 937–954 (2005).
Rahman, M. M. & Laher, I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr. Vasc. Pharmacol. 5, 276–292 (2007).
Zeiher, A. M., Schächinger, V. & Minners, J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation 92, 1094–1100 (1995).
Celermajer, D. S. et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N. Engl. J. Med. 334, 150–154 (1996).
Barua, R. S., Ambrose, J. A., Srivastava, S., DeVoe, M. C. & Eales-Reynolds, L. J. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation 107, 2342–2347 (2003).
Jaimes, E. A., DeMaster, E. G., Tian, R. X. & Raij, L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler. Thromb. Vasc. Biol. 24, 1031–1036 (2004).
Kayyali, U. S. et al. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol. Appl. Pharmacol. 188, 59–68 (2003).
Talukder, M. A. et al. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 300, H388–H396 (2011).
Frey, R. S., Ushio-Fukai, M. & Malik, A. B. NADPH oxidase-dependent signalling in endothelial cells: role in physiology and pathology. Antioxid. Redox Signal. 11, 791–780 (2009).
Li, J. et al. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell 17, 3978–3988 (2006).
Takac, I., Schröder, K. & Brandes, R. P. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr. Hypertens. Rep. 14, 70–78 (2012).
Collins, T. Endothelial nuclear factor-κB and the initiation of the atherosclerotic lesion. Lab. Invest. 68, 499–508 (1993).
Cacciola, R. R., Guarino, F. & Polosa, R. Relevance of endothelial-haemostatic dysfunction in cigarette smoking. Curr. Med. Chem. 14, 1887–1892 (2007).
Jennings, L. K. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 102, 248–257 (2009).
Ruberg, F. L. & Loscalzo, J. Prothrombotic determinants of coronary atherothrombosis. Vasc. Med. 7, 289–299 (2002).
Bernhard, D. et al. Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J. 19, 1096–1107 (2005).
Csordas, A. et al. Cigarette smoke extract induces prolonged endoplasmic reticulum stress and autophagic cell death in human umbilical vein endothelial cells. Cardiovasc. Res. 92, 141–148 (2011).
Falk, E., Shah, P. K. & Fuster, V. Coronary plaque disruption. Circulation 92, 657–671 (1995).
Glaser, R. et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 111, 143–149 (2005).
Wissler, R. W. New insights into the pathogenesis of atherosclerosis as revealed by PDAY. Pathobiological Determinants of Atherosclerosis in Youth. Atherosclerosis 108 (Suppl.), S3–S20 (1994).
Newby, A. C. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc. Med. 17, 253–258 (2007).
Shah, P. K. et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques: potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92, 1565–1569 (1995).
Nelson, K. K. & Melendez, J. A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 37, 768–784 (2004).
Perlstein, T. S. & Lee, R. T. Smoking, metalloproteinases, and vascular disease. Arterioscler. Thromb. Vasc. Biol. 26, 250–256 (2006).
Carty, C. S. et al. Nicotine and cotinine stimulate secretion of basic fibroblast growth factor and affect expression of matrix metalloproteinases in cultured human smooth muscle cells. J. Vasc. Surg. 24, 927–934 (1996).
Liu, P. Y., Chen, J. H., Li, Y. H., Wu, H. L. & Shi, G. Y. Synergistic effect of stromelysin-1 (matrix metallo-proteinase-3) promoter 5A/6A polymorphism with smoking on the onset of young acute myocardial infarction. Thromb. Haemost. 90, 132–139 (2003).
Churg, A. et al. α1-Antitrypsin suppresses TNF-α and MMP-12 production by cigarette smoke-stimulated macrophages. Am. J. Respir. Cell Mol. Biol. 37, 144–151 (2007).
Kangavari, S. et al. Smoking increases inflammation and metalloproteinase expression in human carotid atherosclerotic plaques. J. Cardiovasc. Pharmacol. Ther. 9, 291–298 (2004).
Nordskog, B. K., Blixt, A. D., Morgan, W. T., Fields, W. R. & Hellmann, G. M. Matrix-degrading and pro-inflammatory changes in human vascular endothelial cells exposed to cigarette smoke condensate. Cardiovasc. Toxicol. 3, 101–117 (2003).
Wright, J. L., Tai, H., Wang, R., Wang, X. & Churg, A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-α signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L125–L133 (2007).
Xue, H. et al. Etanercept attenuates short-term cigarette-smoke-exposure-induced pulmonary arterial remodelling in rats by suppressing the activation of TNF-α/NF-κB signal and the activities of MMP-2 and MMP-9. Pulm. Pharmacol. Ther. 25, 208–215 (2012).
Vikman, P., Xu, C. B. & Edvinsson, L. Lipid-soluble cigarette smoking particles induce expression of inflammatory and extracellular-matrix-related genes in rat cerebral arteries. Vasc. Health Risk Manag. 5, 333–341 (2009).
O'Toole, T. E. et al. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol. Appl. Pharmacol. 236, 194–201 (2009).
Lamaître, V., Dabo, A. J. & D'Armiento, J. Cigarette smoke components induce matrix metalloproteinase-1 in aortic endothelial cells through inhibition of mTOR signalling. Toxicol. Sci. 123, 542–549 (2011).
Oikonen, M. et al. Tissue inhibitor of matrix metalloproteinases 4 (TIMP4) in a population of young adults: Relations to cardiovascular risk markers and carotid artery intima–media thickness. The Cardiovascular Risk in Young Finns Study. Scand. J. Clin. Lab. Invest. 72, 540–546 (2012).
Raveendran, M. et al. Cigarette suppresses the expression of P4Hα and vascular collagen production. Biochem. Biophys. Res. Commun. 323, 592–598 (2004).
Jorgensen, L. N., Kallehave, F., Christensen, E., Siana, J. E. & Gottrup, F. Less collagen production in smokers. Surgery 123, 450–455 (1998).
Zhang, K. et al. Interleukin 6 destabilizes atherosclerotic plaques by downregulating prolyl-4-hydroxylase α1 via a mitogen-activated protein kinase and c-Jun pathway. Arch. Biochem. Biophys. 528, 127–133 (2012).
Bermudez, E. A., Rifai, N., Buring, J. E., Manson, J. E. & Ridker, P. M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 22, 1668–1673 (2002).
Grundtman, C., Kreutmayer, S. B., Almanzar, G., Wick, M. C. & Wick, G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 960–968 (2011).
Bouki, K. P. et al. Inflammatory markers and plaque morphology: an optical coherence tomography study. Int. J. Cardiol. 154, 287–292 (2012).
van der Wal, A. C., Becker, A. E., van der Loos, C. M. & Das, P. K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89, 36–44 (1994).
Botti, T. P., Amin, H., Hiltscher, L. & Wissler, R. W. A comparison of the quantitation of macrophage foam cell populations and the extent of apolipoprotein E deposition in developing atherosclerotic lesions in young people: high and low serum thiocyanate groups as an indication of smoking. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Atherosclerosis 124, 191–202 (1996).
Henderson, B. et al. Cigarette smoke is an endothelial stressor and leads to cell cycle arrest. Atherosclerosis 201, 298–305 (2008).
Virmani, R. et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 25, 2054–2061 (2005).
Zhu, B. Q. & Parmley, W. W. Hemodynamic and vascular effects of active and passive smoking. Am. Heart J. 130, 1270–1275 (1995).
Hung, J., Lam, J. Y., Lacoste, L. & Letchacovski, G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation 92, 2432–2436 (1995).
Winniford, M. D. et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation 73, 662–667 (1986).
Wang, L. X. et al. Coronary spasm, a pathogenic trigger of vulnerable plaque rupture. Chin. Med. J. (Engl.) 124, 4071–4178 (2011).
Annex, B. H. et al. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation 91, 619–622 (1995).
Toschi, V. et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 95, 594–599 (1997).
Breitenstein, A., Camici, G. G. & Tanner, F. C. Tissue factor: beyond coagulation in the cardiovascular system. Clin. Sci. (Lond.) 118, 159–172 (2010).
Sambola, A. et al. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation 107, 973–977 (2003).
Li, M., Yu, D., Williams, K. J. & Liu, M. L. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler. Thromb. Vasc. Biol. 30, 1818–1824 (2010).
Cirillo, P. et al. Nicotine induces tissue factor expression in cultured endothelial and smooth muscle cells. J. Thromb. Haemost. 4, 453–458 (2006).
Matetzky, S. et al. Smoking increases tissue factor expression in atherosclerotic plaques: implications for plaque thrombogenicity. Circulation 102, 602–604 (2000).
Hölschermann, H. et al. Monocyte tissue factor expression is enhanced in women who smoke and use oral contraceptives. Thromb. Haemost. 82, 1614–1620 (1999).
Heiss, C. et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 51, 1760–1771 (2008).
Kreutmayer, S. B. et al. Dynamics of heat shock protein 60 in endothelial cells exposed to cigarette smoke extract. J. Mol. Cell. Cardiol. 51, 777–780 (2011).
Wickenden, J. A. et al. Cigarette smoke prevents apoptosis through inhibition of caspase activation and induces necrosis. Am. J. Respir. Cell Mol. Biol. 29, 562–570 (2003).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Thaulow, E., Erikssen, J., Sandvik, L., Stormorken, H. & Cohn, P. F. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation 84, 613–617 (1991).
Law, M. R. & Wald, N. J. Environmental tobacco smoke and ischemic heart disease. Prog. Cardiovasc. Dis. 46, 31–38 (2003).
Davis, J. W., Shelton, L., Eigenberg, D. A., Hignite, C. E. & Watanabe, I. S. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin. Pharmacol. Ther. 37, 529–533 (1985).
Imaizumi, T. et al. Effect of cigarette smoking on the levels of platelet-activating factor-like lipid(s) in plasma lipoproteins. Atherosclerosis 87, 47–55 (1991).
Fusegawa, Y., Goto, S., Handa, S., Kawada, T. & Ando, Y. Platelet spontaneous aggregation in platelet-rich plasma is increased in habitual smokers. Thromb. Res. 93, 271–278 (1999).
Levine, P. H. An acute effect of cigarette smoking on platelet function: a possible link between smoking and arterial thrombosis. Circulation 48, 619–623 (1973).
Glynn, M. F., Mustard, J. F., Buchanan, M. R. & Murphy, E. A. Cigarette smoking and platelet aggregation. Can. Med. Assoc. J. 95, 549–553 (1966).
Caponnetto, P. et al. Circulating endothelial-coagulative activation markers after smoking cessation: a 12-month observational study. Eur. J. Clin. Invest. 41, 616–626 (2011).
Blache, D. Involvement of hydrogen and lipid peroxides in acute tobacco smoking-induced platelet hyperactivity. Am. J. Physiol. 268, H679–H685 (1995).
Lupia, E. et al. Thrombopoietin contributes to enhanced platelet activation in cigarette smokers. Atherosclerosis 210, 314–319 (2010).
Yarlioglues, M. et al. The acute effects of passive smoking on mean platelet volume in healthy volunteers. Angiology 63, 353–357 (2012).
Sinzinger, H. & Kefalides, A. Passive smoking severely decreases platelet sensitivity to antiaggregatory prostaglandins. Lancet 2, 392–393 (1982).
Schmid, P. et al. Passive smoking and platelet thromboxane. Thromb. Res. 81, 451–460 (1996).
Tell, G. S., Grimm, R. H., Vellar, O. D. & Theodorsen, L. The relationship of white cell count, platelet count, and hematocrit to cigarette smoking in adolescents: the Oslo Youth Study. Circulation 72, 971–974 (1985).
Podrez, E. A. et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 13, 1086–1095 (2007).
Miyaura, S., Eguchi, H. & Johnston, J. M. Effect of a cigarette smoke extract on the metabolism of the proinflammatory autacoid, platelet-activating factor. Circ. Res. 70, 341–347 (1992).
Marathe, G. K., Prescott, S. M., Zimmerman, G. A. & McIntyre, T. M. Oxidized LDL contains inflammatory PAF-like phospholipids. Trends Cardiovasc. Med. 11, 139–142 (2001).
Togna, A. R., Latina, V., Orlando, R. & Togna, G. I. Cigarette smoke inhibits adenine nucleotide hydrolysis by human platelets. Platelets 19, 537–542 (2008).
Ichiki, K., Ikeda, H., Haramaki, N., Ueno, T. & Imaizumi, T. Long-term smoking impairs platelet-derived nitric oxide release. Circulation 94, 3109–3114 (1996).
Haramaki, N. et al. Long-term smoking causes nitroglycerin resistance in platelets by depletion of intraplatelet glutathione. Arterioscler. Thromb. Vasc. Biol. 21, 1852–1856 (2001).
Takajo, Y., Ikeda, H., Haramaki, N., Murohara, T. & Imaizumi, T. Augmented oxidative stress of platelets in chronic smokers: mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. J. Am. Coll. Cardiol. 38, 1320–1327 (2001).
Della Corte, A. et al. Platelet proteome in healthy volunteers who smoke. Platelets 23, 91–105 (2012).
Hennan, J. K. et al. Effects of selective cyclooxygenase-2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation 104, 820–825 (2001).
FitzGerald, G. A., Oates, J. A. & Nowak, J. Cigarette smoking and hemostatic function. Am. Heart J. 115, 267–271 (1988).
Reinders, J. H., Brinkman, H. J., van Mourik, J. A. & de Groot, P. G. Cigarette smoke impairs endothelial cell prostacyclin production. Arteriosclerosis 6, 15–23 (1986).
Hioki, H. et al. Acute effects of cigarette smoking on platelet-dependent thrombin generation. Eur. Heart J. 22, 56–61 (2001).
Kimura, S., Nishinaga, M., Ozawa, T. & Shimada, K. Thrombin generation as an acute effect of cigarette smoking. Am. Heart J. 128, 7–11 (1994).
Renaud, S., Blache, D., Dumont, E., Thevenon, C. & Wissendanger, T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin. Pharmacol. Ther. 36, 389–395 (1984).
Watts, D. T. The effect of nicotine and smoking on the secretion of epinephrine. Ann. NY Acad. Sci. 90, 74–80 (1960).
Lande, K., Gjesdal, K., Fønstelien, E., Kjeldsen, S. E. & Eide, I. Effects of adrenaline infusion on platelet number, volume and release reaction. Thromb. Haemost. 54, 450–453 (1985).
Harding, S. A. et al. Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation 109, 1926–1929 (2004).
Sithu, S. D. et al. Exposure to acrolein by inhalation causes platelet activation. Toxicol. Appl. Pharmacol. 248, 100–110 (2010).
Andrè, E. et al. Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 (2008).
Selley, M. L., Bartlett, M. R., McGuiness, J. A. & Ardlie, N. G. Effects of acrolein on human platelet aggregation. Chem. Biol. Interact. 76, 101–109 (1990).
Ambrose, J. A. & Barua, R. S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol. 43, 1731–1737 (2004).
Miller, G. J., Bauer, K. A., Cooper, J. A. & Rosenberg, R. D. Activation of the coagulant pathway in cigarette smokers. Thromb. Haemost. 79, 549–553 (1998).
Markuljak, I., Ivankova, J. & Kubisz, P. Thrombomodulin and von Willebrand factor in smokers and during smoking. Nouv. Rev. Fr. Hematol. 37, 137–139 (1995).
Raza, S. L., Nehring, L. C., Shapiro, S. D. & Cornelius, L. A. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J. Biol. Chem. 275, 41243–41250 (2000).
Kwaan, H. C. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clin. Hemorheol. Microcirc. 44, 167–176 (2010).
Heinrich, J., Balleisen, L., Schulte, H., Assmann, G. & van de Loo, J. Fibrinogen and factor VII in the prediction of coronary PROCAM study in healthy men. Arterioscler. Thromb. 14, 54–59 (1994).
Tuut, M. & Hense, H. W. Smoking, other risk factors and fibrinogen levels: evidence of effect modification. Ann. Epidemiol. 11, 232–238 (2001).
Hunter, K. A., Garlick, P. J., Broom, I., Anderson, S. E. & McNurlan, M. A. Effects of smoking and abstention from smoking on fibrinogen synthesis in humans. Clin. Sci. (Lond.) 100, 459–465 (2001).
Stone, M. C. & Thorp, J. M. Plasma fibrinogen--a major coronary risk factor. J. R. Coll. Gen. Pract. 35, 565–569 (1985).
Tapson, V. F. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2, 71–77 (2005).
Barbash, G. I. et al. Significance of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: experience gleaned from the International Tissue Plasminogen Activator/Streptokinase Mortality trial. Circulation 87, 53–58 (1993).
Barbash, G. I., White, H. D., Modan, M. & Van der Werf, F. Smoking and acute myocardial infarction. Circulation 87, 1427–1428 (1993).
Kirtane, A. J. et al. Association of smoking with improved myocardial perfusion and the angiographic characterization of myocardial tissue perfusion after fibrinolytic therapy for ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 45, 321–323 (2005).
Barua, R. S. et al. Acute cigarette smoke exposure reduces clot lysis--association between altered fibrin architecture and the response to t-PA. Thromb. Res. 126, 426–430 (2010).
Pretorius, E., Oberholzer, H. M., van der Spuy, W. J. & Meiring, J. H. Smoking and coagulation: the sticky fibrin phenomenon. Ultrastruct. Pathol. 34, 236–239 (2010).
Shacter, E., Williams, J. A., Lim, M. & Levine, R. L. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic. Biol. Med. 17, 429–437 (1994).
Parahuelva, M. S. et al. Nicotine modulation of factor VII activating protease (FSAP) expression in human monocytes. J. Atheroscler. Thromb. 19, 962–969 (2012).
van Wersch, J. W., Vooijs, M. E. & Ubachs, J. M. Coagulation factor XIII in pregnant smokers and non-smokers. Int. J. Clin. Lab. Res. 27, 68–71 (1997).
Allen, R. A., Kluft, C. & Brommer, E. J. Effect of chronic smoking on fibrinolysis. Arteriosclerosis 5, 443–450 (1985).
Newby, D. E. et al. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation 103, 1936–1941 (2001).
Newby, D. E. et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 99, 1411–1415 (1999).
Kaehler, J. et al. Impaired capacity for acute endogenous fibrinolysis in smokers is restored by ascorbic acid. Free Radic. Biol. Med. 44, 315–321 (2008).
Barua, R. S., Ambrose, J. A., Saha, D. C. & Eales-Reynolds, L. J. Smoking is associated with altered endothelial-derived fibrinolytic and antithrombotic factors: an in vitro demonstration. Circulation 106, 905–908 (2002).
Pellegrini, M. P., Newby, D. E., Maxwell, S. & Webb, D. J. Short-term effects of transdermal nicotine on acute tissue plasminogen activator release in vivo in man. Cardiovasc. Res. 52, 321–327 (2001).
Zidovetzki, R., Chen, P., Fisher, M., Hofman, F. M. & Faraci, F. M. Nicotine increases plasminogen activator inhibitor-1 production by human brain endothelial cells via protein kinase C-associated pathway. Stroke 30, 651–655 (1999).
Haire, W. D., Goldsmith, J. C. & Rasmussen, J. Abnormal fibrinolysis in healthy male cigarette smokers: role of plasminogen activator inhibitors. Am. J. Hematol. 31, 36–40 (1989).
Simpson, A. J., Gray, R. S., Moore, N. R. & Booth, N. A. The effects of chronic smoking on the fibrinolytic potential of plasma and platelets. Br. J. Haematol. 97, 208–13 (1997).
Margaglione, M. et al. PAI-1 plasma levels in a general population without clinical evidence of atherosclerosis: relation to environmental and genetic determinants. Arterioscler. Thromb. Vasc. Biol. 18, 562–567 (1998).
Tzoulaki, I. et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation 115, 2119–2127 (2007).
Kotani, K., Inata, A. & Araga, S. Hemorheology by microchannel method in males with metabolic syndrome. Arch. Med. Res. 38, 463–464 (2007).
Lowe, G. D., Drummond, M. M., Forbes, C. D. & Barbenel, J. C. The effects of age and cigarette-smoking on blood and plasma viscosity in men. Scott. Med. J. 25, 13–17 (1980).
Yarnell, J. W. et al. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease: the Caerphilly and Speedwell collaborative heart disease studies. Circulation 83, 836–844 (1991).
Haustein, K. O., Krause, J., Haustein, H., Rasmussen, T. & Cort, N. Effects of cigarette smoking or nicotine replacement on cardiovascular risk factors and parameters of haemorheology. J. Intern. Med. 252, 130–139 (2002).
Shimada, S. et al. High blood viscosity is closely associated with cigarette smoking and markedly reduced by smoking cessation. Circ. J. 75, 185–189 (2011).
Price, J. F. et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur. Heart J. 20, 344–353 (1999).
Ernst, E. Haemorheological consequences of chronic cigarette smoking. J. Cardiovasc. Risk 2, 435–439 (1995).
Wang, X. L., Sim, A. S., Badenhop, R. F., Mccredie R. M. & Wilcken, D. E. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxidase gene. Nat. Med. 2, 41–45 (1996).
Ragia, G. et al. Endothelial nitric oxide synthase gene polymorphisms -786T>C and 894G>T in coronary artery bypass graft surgery patients. Hum. Genomics 4, 375–383 (2010).
Dzida, G., Sobstyl, J., Pužniak A., Prystupa, A. & Mosiewicz, J. Impact of smoking status on particular genetic polymorphisms associations with cardiovascular diseases. J. Preclin. Clin. Res. 6, 31–34 (2012).
Lee, C. R. et al. NOS3 polymorphisms, cigarette smoking, and cardiovascular disease. Pharmacogenet. Genomics 16, 891–899 (2006).
Nasreen, S. et al. T-786C polymorphism in endothelial NO synthetase gene affects cerebral circulation in smokers: possible gene-environmental interaction. Arterioscler. Thromb. Vasc. Biol. 22, 605–610 (2002).
Yin, R.-X. et al. Interactions of several lipid-related gene polymorphisms and cigarette smoking on blood pressure levels. Int. J. Biol. Sci. 8, 685–696 (2012).
Sen-Banerjee, S., Siles, X. & Campos, H. Tobacco smoke modifies association between Glu-Arg192 polymorphism of human paraxonase gene and risk of myocardial infarction. Atheroscler. Thromb. Vasc. Biol. 20, 2120–2126 (2000).
Frey, P. et al. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL] trials). Am. J. Cardiol. 107, 145–150 (2011).
Acknowledgements
D. Bernhard is supported by the Austrian National Bank (Project 14745).
Author information
Authors and Affiliations
Contributions
Both authors were involved in all stages of the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Csordas, A., Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 10, 219–230 (2013). https://doi.org/10.1038/nrcardio.2013.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2013.8
This article is cited by
-
Gut microbial metabolites reveal diet-dependent metabolic changes induced by nicotine administration
Scientific Reports (2024)
-
Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Non-invasive biomarkers for early diagnosis of pancreatic cancer risk: metabolite genomewide association study based on the KCPS-II cohort
Journal of Translational Medicine (2023)
-
Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures
GeroScience (2023)
-
Cardiovascular Profiles of Younger and Older Coronary Artery Disease Patients in Asian and Western Regions
Current Epidemiology Reports (2023)