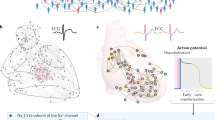
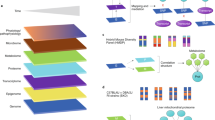
Abstract
Common cardiovascular diseases, such as atherosclerosis and congestive heart failure, are exceptionally complex, involving a multitude of environmental and genetic factors that often show nonlinear interactions as well as being highly dependent on sex, age, and even the maternal environment. Although focused, reductionistic approaches have led to progress in elucidating the pathophysiology of cardiovascular diseases, such approaches are poorly powered to address complex interactions. Over the past decade, technological advances have made it possible to interrogate biological systems on a global level, raising hopes that, in combination with computational approaches, it may be possible to more fully address the complexities of cardiovascular diseases. In this Review, we provide an overview of such systems-based approaches to cardiovascular disease and discuss their translational implications.
Key Points
-
The cardiovascular system is a complex network of organs and cell types, each with specialized, but highly coordinated, functions; therefore, cardiovascular disease is complex in etiology and manifestation
-
A systems-based approach aims to reveal the architecture and the emerging properties of a complex network by uncovering the relationships among the constituents and establishing global governing principles
-
Current advances in bioinformatics, genomics, proteomics, and metabolomics offer an excellent opportunity to use systems-based analysis to dissect complex networks involved in cardiovascular physiology and diseases
-
In the study of cardiovascular diseases, systems biology compliments genetic analyses, such as genome-wide association studies, by establishing the underlying mechanisms and the functional significance of the candidate genes
-
'Systems genetics' is a new approach based on the systems-based analysis of genetic variants and phenotypic spectra at various levels, spanning from gene expression to organ physiology
-
By establishing the molecular components and gene networks for the cardiovascular system, systems biology can help to develop effective and personalized diagnostic tools and therapies for cardiovascular diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schunkert, H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011).
Barabasi, A. L. Linked: The new science of networks (Penguin Books, London, 2002).
Bousquet, J. et al. Systems medicine and integrated care to combat chronic noncommunicable diseases. Genome Med. 3, 43 (2011).
Ideker, T., Galitski, T. & Hood, L. A new approach to decoding life: systems biology. Annu. Rev. Genomics Hum. Genet. 2, 343–372 (2001).
Nadeau, J. H. & Subramaniam, S. Systems biology and medicine: a new take on an old paradigm. Wiley Interdiscip. Rev. Syst. Biol. Med. 1, 1–3 (2009).
Nadeau, J. H. & Subramaniam, S. Systems biology—old wine in a new bottle or is the bottle changing the wine? Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 1–2 (2010).
Schadt, E. E. Molecular networks as sensors and drivers of common human diseases. Nature 461, 218–223 (2009).
Schadt, E. E. & Lum, P. Y. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Reverse engineering gene networks to identify key drivers of complex disease phenotypes. J. Lipid Res. 47, 2601–2613 (2006).
Noble, D. The surprising heart: a review of recent progress in cardiac electrophysiology. J. Physiol. 353, 1–50 (1984).
Noble, D. Modeling the heart—from genes to cells to the whole organ. Science 295, 1678–1682 (2002).
Noble, D. The music of life: biology beyond genes (Oxford University Press, Oxford, 2006).
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000).
Mudd, J. O. & Kass, D. A. Tackling heart failure in the twenty-first century. Nature 451, 919–928 (2008).
Bondarenko, V. E., Szigeti, G. P., Bett, G. C., Kim, S. J. & Rasmusson, R. L. Computer model of action potential of mouse ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 287, H1378–1403 (2004).
Greenstein, J. L. & Winslow, R. L. An integrative model of the cardiac ventricular myocyte incorporating local control of Ca2 release. Biophys. J. 83, 2918–2945 (2002).
Shannon, T. R., Wang, F., Puglisi, J., Weber, C. & Bers, D. M. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys. J. 87, 3351–3371 (2004).
Weiss, J. N. et al. From pulsus to pulseless: the saga of cardiac alternans. Circ. Res. 98, 1244–1253 (2006).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Lusis, A. J. & Weiss, J. N. Cardiovascular networks: systems-based approaches to cardiovascular disease. Circulation 121, 157–170 (2010).
Newman, M. E. J. Networks: an introduction (Oxford University Press, Oxford, 2010).
Shipley, B. Cause and correlation: a user's guide to path analysis, structural equations and causal inference (Cambridge University Press, Cambridge, 2002).
Subramaniam, S. & Nadeau, J. H. Systems medicine—viewed through the real and computing lenses. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 383–384 (2010).
Langfelder, P. & Horvath, S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst. Biol. 1, 54 (2007).
Ferrell, J. E. Jr, Tsai, T. Y. & Yang, Q. Modeling the cell cycle: why do certain circuits oscillate? Cell 144, 874–885 (2011).
Yosef, N. & Regev, A. Impulse control: temporal dynamics in gene transcription. Cell 144, 886–896 (2011).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Giallourakis, C., Henson, C., Reich, M., Xie, X. & Mootha, V. K. Disease gene discovery through integrative genomics. Annu Rev. Genomics Hum. Genet. 6, 381–406 (2005).
Stoll, M. et al. A genomic-systems biology map for cardiovascular function. Science 294, 1723–1726 (2001).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Keller, M. P. & Attie, A. D. Physiological insights gained from gene expression analysis in obesity and diabetes. Annu. Rev. Nutr. 30, 341–364 (2010).
Su, W. L., Kleinhanz, R. R. & Schadt, E. E. Characterizing the role of miRNAs within gene regulatory networks using integrative genomics techniques. Mol. Syst. Biol. 7, 490 (2011).
Zhong, H., Yang, X., Kaplan, L. M., Molony, C. & Schadt, E. E. Integrating pathway analysis and genetics of gene expression for genome-wide association studies. Am. J. Hum. Genet. 86, 581–591 (2010).
Deng, M. C. et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am. J. Transplant. 6, 150–160 (2006).
Peri, S. et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 13, 2363–2371 (2003).
Gerszten, R. E., Asnani, A. & Carr, S. A. Status and prospects for discovery and verification of new biomarkers of cardiovascular disease by proteomics. Circ. Res. 109, 463–474 (2011).
Lewis, G. D. & Gerszten, R. E. Toward metabolomic signatures of cardiovascular disease. Circ. Cardiovasc. Genet. 3, 119–121 (2010).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Small, K. S. et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 43, 561–564 (2011).
Lander, E. S. Genome-sequencing anniversary. The accelerator. Science 331, 1024 (2011).
Neto, E. C., Keller, M. P., Attie, A. D. & Yandell, B. S. Causal graphical models in systems genetics: a unified framework for joint inference of causal network and genetic architecture for correlated phenotypes. Ann. Appl. Stat. 4, 320–339 (2010).
Vidal, M., Cusick, M. E. & Barabasi, A. L. Interactome networks and human disease. Cell 144, 986–998 (2011).
Barabasi, A. L. & Bonabeau, E. Scale-free networks. Sci. Am. 288, 60–69 (2003).
Ravasz, E., Somera, A. L., Mongru, D. A., Oltvai, Z. N. & Barabasi, A. L. Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555 (2002).
Jeong, H., Tombor, B., Albert, R., Oltvai, Z. N. & Barabasi, A. L. The large-scale organization of metabolic networks. Nature 407, 651–654 (2000).
Albert, R., Jeong, H. & Barabasi, A. L. Error and attack tolerance of complex networks. Nature 406, 378–382 (2000).
Barabasi, A. L. Scale-free networks: a decade and beyond. Science 325, 412–413 (2009).
Cai, C. et al. Is human blood a good surrogate for brain tissue in transcriptional studies? BMC Genomics 11, 589 (2010).
Miller, J. A., Horvath, S. & Geschwind, D. H. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc. Natl Acad. Sci. USA 107, 12698–12703 (2010).
van Nas, A. et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology 150, 1235–1249 (2009).
Langfelder, P., Luo, R., Oldham, M. C. & Horvath, S. Is my network module preserved and reproducible? PLoS Comput. Biol. 7, e1001057 (2011).
Loscalzo, J., Kohane, I. & Barabasi, A. L. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol. Syst. Biol. 3, 124 (2007).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 (2006).
Libby, P. & Theroux, P. Pathophysiology of coronary artery disease. Circulation 111, 3481–3488 (2005).
Jessup, M. & Brozena, S. Heart failure. N. Engl. J. Med. 348, 2007–2018 (2003).
Creemers, E. E., Wilde, A. A. & Pinto, Y. M. Heart failure: advances through genomics. Nat. Rev. Genet. 12, 357–362 (2011).
Romanoski, C. E. et al. Systems genetics analysis of gene-by-environment interactions in human cells. Am. J. Hum. Genet. 86, 399–410 (2010).
Ramsey, S. A. et al. Uncovering a macrophage transcriptional program by integrating evidence from motif scanning and expression dynamics. PLoS Comput. Biol. 4, e1000021 (2008).
Ideker, T., Dutkowski, J. & Hood, L. Boosting signal-to-noise in complex biology: prior knowledge is power. Cell 144, 860–863 (2011).
Lander, A. D. Pattern, growth, and control. Cell 144, 955–969 (2011).
Becker, L. et al. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 11, 125–135 (2010).
Brehme, M. & Vidal, M. A global protein-lipid interactome map. Mol. Syst. Biol. 6, 443 (2010).
Nadeau, J. H. et al. Pleiotropy, homeostasis, and functional networks based on assays of cardiovascular traits in genetically randomized populations. Genome Res. 13, 2082–2091 (2003).
Nadeau, J. H. & Dudley, A. M. Genetics. Systems genetics. Science 331, 1015–1016 (2011).
Ghazalpour, A. et al. Genomic analysis of metabolic pathway gene expression in mice. Genome Biol. 6, R59 (2005).
Chen, Y. et al. Variations in DNA elucidate molecular networks that cause disease. Nature 452, 429–435 (2008).
Ghazalpour, A. et al. Integrating genetic and network analysis to characterize genes related to mouse weight. PLoS Genet. 2, e130 (2006).
Keller, M. P. et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 18, 706–716 (2008).
Meng, H. et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc. Natl Acad. Sci. USA 104, 4530–4535 (2007).
Mungrue, I. N., Pagnon, J., Kohannim, O., Gargalovic, P. S. & Lusis, A. J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J. Immunol. 182, 466–476 (2009).
Schadt, E. E. et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat. Genet. 37, 710–717 (2005).
Wang, S. S. et al. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ. Res. 101, e11–e30 (2007).
Yang, X. et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat. Genet. 41, 415–423 (2009).
Hagg, S. et al. Multi-organ expression profiling uncovers a gene module in coronary artery disease involving transendothelial migration of leukocytes and LIM domain binding 2: the Stockholm Atherosclerosis Gene Expression (STAGE) study. PLoS Genet. 5, e1000754 (2009).
Dobrin, R. et al. Multi-tissue coexpression networks reveal unexpected subnetworks associated with disease. Genome Biol. 10, R55 (2009).
Beaumont, M. A. & Rannala, B. The Bayesian revolution in genetics. Nat. Rev. Genet. 5, 251–261 (2004).
Needham, C. J., Bradford, J. R., Bulpitt, A. J. & Westhead, D. R. A primer on learning in Bayesian networks for computational biology. PLoS Comput. Biol. 3, e129 (2007).
Emilsson, V. et al. Genetics of gene expression and its effect on disease. Nature 452, 423–428 (2008).
Ferrara, C. T. et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 4, e1000034 (2008).
Gargalovic, P. S. et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl Acad. Sci. USA 103, 12741–12746 (2006).
Romanoski, C. E. et al. Network for activation of human endothelial cells by oxidized phospholipids: a critical role of heme oxygenase 1. Circ. Res. 109, e27–e41 (2011).
Petretto, E. et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat. Genet. 40, 546–552 (2008).
Sarwar, R. & Cook, S. A. Genomic analysis of left ventricular remodeling. Circulation 120, 437–444 (2009).
Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 43, 339–344 (2011).
Manolio, T. A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 363, 166–176 (2010).
Musunuru, K. et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466, 714–719 (2010).
Goh, K. I. et al. The human disease network. Proc. Natl Acad. Sci. USA 104, 8685–8690 (2007).
Horwitz, P. A. et al. Detection of cardiac allograft rejection and response to immunosuppressive therapy with peripheral blood gene expression. Circulation 110, 3815–3821 (2004).
Pham, M. X. et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N. Engl. J. Med. 362, 1890–1900 (2010).
Eagle, K. A. et al. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation 121, 1447–1454 (2010).
Bloss, C. S., Schork, N. J. & Topol, E. J. Effect of direct-to-consumer genomewide profiling to assess disease risk. N. Engl. J. Med. 364, 524–534 (2011).
Ginsburg, G. S., Donahue, M. P. & Newby, L. K. Prospects for personalized cardiovascular medicine: the impact of genomics. J. Am. Coll. Cardiol. 46, 1615–1627 (2005).
Nadeau, J. H. & Topol, E. J. The genetics of health. Nat. Genet. 38, 1095–1098 (2006).
Anderson, J. L. et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 116, 2563–2570 (2007).
Krynetskiy, E. & McDonnell, P. Building individualized medicine: prevention of adverse reactions to warfarin therapy. J. Pharmacol. Exp. Ther. 322, 427–434 (2007).
Connolly, S. J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151 (2009).
Baudhuin, L. M. et al. Relation of ADRB1, CYP2D6, and UGT1A1 polymorphisms with dose of, and response to, carvedilol or metoprolol therapy in patients with chronic heart failure. Am. J. Cardiol. 106, 402–408 (2010).
Sehnert, A. J. et al. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J. Am. Coll. Cardiol. 52, 644–651 (2008).
Sehrt, D., Meineke, I., Tzvetkov, M., Gultepe, S. & Brockmöller, J. Carvedilol pharmacokinetics and pharmacodynamics in relation to CYP2D6 and ADRB pharmacogenetics. Pharmacogenomics 12, 783–795 (2011).
SEARCH Collaborative Group et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 359, 789–799 (2008).
Chasman, D. I. et al. Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 291, 2821–2827 (2004).
Thompson, J. F. et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics J. 5, 352–358 (2005).
Shu, Y. et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc. Natl Acad. Sci. USA 100, 5902–5907 (2003).
Reitman, M. L. & Schadt, E. E. Pharmacogenetics of metformin response: a step in the path toward personalized medicine. J. Clin. Invest. 117, 1226–1229 (2007).
Hughes, B. 2009 FDA drug approvals. Nat. Rev. Drug Discov. 9, 89–92 (2010).
Weiss, J. N., Garfinkel, A. & Chen, P. S. Novel approaches to identifying antiarrhythmic drugs. Trends Cardiovasc. Med. 13, 326–330 (2003).
Garfinkel, A. et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc. Natl Acad. Sci. USA 97, 6061–6066 (2000).
Baggs, J. E., Hughes, M. E. & Hogenesch, J. B. The network as the target. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 127–133 (2010).
Behrends, C., Sowa, M. E., Gygi, S. P. & Harper, J. W. Network organization of the human autophagy system. Nature 466, 68–76 (2010).
Wang, Z. V., Rothermel, B. A. & Hill, J. A. Autophagy in hypertensive heart disease. J. Biol. Chem. 285, 8509–8514 (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article, including researching data, discussion of content, and writing, reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
MacLellan, W., Wang, Y. & Lusis, A. Systems-based approaches to cardiovascular disease. Nat Rev Cardiol 9, 172–184 (2012). https://doi.org/10.1038/nrcardio.2011.208
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.208
This article is cited by
-
A urinary peptidomics approach for early stages of cardiovascular disease risk: The African-PREDICT study
Hypertension Research (2023)
-
High sodium intake and sodium to potassium ratio may be linked to subsequent increase in vascular damage in adults aged 40 years and older: the Korean multi-rural communities cohort (MRCohort)
European Journal of Nutrition (2019)
-
New perspectives: systems medicine in cardiovascular disease
BMC Systems Biology (2018)
-
A data-driven, knowledge-based approach to biomarker discovery: application to circulating microRNA markers of colorectal cancer prognosis
npj Systems Biology and Applications (2018)
-
Analysis of Combined Transcriptomes Identifies Gene Modules that Differentially Respond to Pathogenic Stimulation of Vascular Smooth Muscle and Endothelial Cells
Scientific Reports (2018)