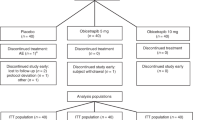
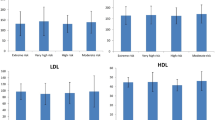
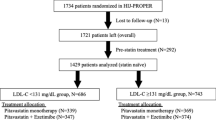
Abstract
A continuous, graded relationship exists between LDL cholesterol (LDL-C) levels and risk of cardiovascular disease (CVD). This finding has been confirmed at progressively lower levels of LDL-C by results from clinical trials of therapies, particularly high-potency statins, which provide increasingly greater reductions in LDL-C levels. On the basis of this clinical trial evidence, progressively lower LDL-C goals for increasing numbers of patients, stratified by absolute CVD risk, have been recommended in guidelines for cholesterol management and CVD prevention. Some notable exceptions have been made, however, such as patients with end-stage renal disease or heart failure. To achieve low LDL-C goals, statins are first-line pharmacological therapy and can be combined with other agents to provide additional reductions in LDL-C levels as well as improvements in other lipoprotein fractions. Investigational agents that reduce LDL-C levels by other mechanisms are under development and could provide additional therapeutic strategies to achieve optimal LDL-C levels. These agents could be particularly appropriate for patients with severely elevated LDL-C levels, such as those with genetic dyslipidemia, for whom maximal drug therapy is insufficient to reduce LDL-C concentrations to recommended levels.
Key Points
-
Results from clinical trials have confirmed the persistence of the relationship between LDL cholesterol (LDL-C) and cardiovascular disease (CVD) at progressively lower levels of LDL-C
-
On the basis of available clinical trial evidence, guidelines for cholesterol management and CVD prevention have recommended progressively lower LDL-C goals
-
Statins are first-line drug therapy for reducing LDL-C levels in most patients
-
For patients in whom statin monotherapy does not reduce LDL-C concentrations to recommended levels, combination therapy with other lipid-regulating agents can further reduce the levels of LDL-C and other lipid fractions
-
Investigational agents that reduce LDL-C by different mechanisms than conventional therapy could provide additional strategies for reduction of LDL-C levels
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation 106, 3143–3421 (2002).
Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Graham, I. et al. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur. J. Cardiovasc. Prev. Rehabil. 14 (Suppl. 2), S1–S113 (2007).
Genest, J. et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can. J. Cardiol. 25, 567–579 (2009).
Di Angelantonio, E. et al. for the Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Sharrett, A. R. et al. for the Atherosclerosis Risk in Communities Study Group. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104, 1108–1113 (2001).
Cohen, J. C., Boerwinkle, E., Mosley, T. H., Jr & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002).
Cannon, C. P. et al. for the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Comparison of intensive and moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004).
Sever, P. S. et al. for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicenter randomised controlled trial. Lancet 361, 1149–1158 (2003).
Shepherd, J. et al. for the PROSPER study group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360, 1623–1630 (2002).
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 288, 2998–3007 (2002).
Grundy, S. M. et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110, 227–239 (2004).
LaRosa, J. C. et al. for the Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N. Engl. J. Med. 352, 1425–1435 (2005).
Pedersen, T. R. et al. for the IDEAL Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 294, 2437–2445 (2005).
Smith, S. C., Jr. et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 113, 2363–2372 (2006).
Brunzell, J. D. et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 51, 1512–1524 (2008).
Ridker, P. M. et al. for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Ridker, P. M. et al. for the JUPITER Trial Study Group. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet 376, 333–339 (2010).
Jones, P. H. et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin. Ther. 26, 1388–1399 (2004).
Pasternak, R. C. et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J. Am. Coll. Cardiol. 40, 567–572 (2002).
Arnaboldi, L. & Corsini, A. Do structural differences in statins correlate with clinical efficacy? Curr. Opin. Lipidol. 21, 298–304 (2010).
Jones, P. H. et al. for the STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial). Am. J. Cardiol. 92, 152–160 (2003).
Saito, Y. Critical appraisal of the role of pitavastatin in treating dyslipidemias and achieving lipid goals. Vasc. Health Risk. Manag. 5, 921–936 (2009).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248 (2005).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Kjekshus, J. et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 357, 2248–2261 (2007).
GISSI-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372, 1231–1239 (2008).
Carlson, L. A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 258, 94–114 (2005).
Bays, H. E. & Ballantyne, C. What's the deal with niacin development: is laropiprant add-on therapy a winning strategy to beat a straight flush? Curr. Opin. Lipidol. 20, 467–476 (2009).
Corsini, A., Windler, E. & Farnier, M. Colesevelam hydrochloride: usefulness of a specifically engineered bile acid sequestrant for lowering LDL-cholesterol. Eur. J. Cardiovasc. Prev. Rehabil. 16, 1–9 (2009).
Zieve, F. J., Kalin, M. F., Schwartz, S. L., Jones, M. R. & Bailey, W. L. Results of the Glucose-Lowering Effect of WelChol Study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin. Ther. 29, 74–83 (2007).
Bays, H. E., Davidson, M., Jones, M. R. & Abby, S. L. Effects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemia. Am. J. Cardiol. 97, 1198–1205 (2006).
Hou, R. & Goldberg, A. C. Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety. Endocrinol. Metab. Clin. North Am. 38, 79–97 (2009).
Sudhop, T. et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106, 1943–1948 (2002).
Ballantyne, C. M. et al. for the EXPLORER Study Investigators. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am. J. Cardiol. 99, 673–680 (2007).
Califf, R. M. et al. An update on the IMProved reduction of outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) design. Am. Heart J. 159, 705–709 (2010).
Davidson, M. H. Statin/fibrate combination in patients with metabolic syndrome or diabetes: evaluating the risks of pharmacokinetic drug interactions. Expert Opin. Drug Saf. 5, 145–156 (2006).
Goldberg, A. C. et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia. Am. J. Cardiol. 103, 515–522 (2009).
Staels, B. et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98, 2088–2093 (1998).
Jones, P. H. et al. Efficacy and safety of fenofibric acid co-administered with low- or moderate-dose statin in patients with mixed dyslipidemia and type 2 diabetes mellitus: results of a pooled subgroup analysis from three randomized, controlled, double-blind trials. Am. J. Cardiovasc. Drugs 10, 73–84 (2010).
Manninen, V. et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation 85, 37–45 (1992).
Rubins, H. B. et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med. 341, 410–418 (1999).
BIP Study Group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 102, 21–27 (2000).
IELD Study Investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9,795 people with type 2 diabetes mellitus (the FIELD study). Lancet 366, 1849–1861 (2005).
ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
Harris, W. S. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J. Lipid Res. 30, 785–807 (1989).
Davidson, M. H. et al. for the COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 29, 1354–1367 (2007).
McKenney, J. M. & Sica, D. Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia. Am. J. Health Syst. Pharm. 64, 595–605 (2007).
Yokoyama, M. et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 (2007).
Brown, M. S., Hobbs, H. H. & Goldstein, J. L. in Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R., Sly, W. S. & Childs, B.) Ch. 120 (McGraw-Hill, New York, 2001) [online], (2001).
Myant, N. B. Familial defective apolipoprotein B-100: a review, including some comparisons with familial hypercholesterolaemia. Atherosclerosis 104, 1–18 (1993).
Tarugi, P. et al. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis 195, e19–e27 (2007).
Sankatsing, R. R. et al. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 25, 1979–1984 (2005).
Crooke, S. T. Progress in antisense technology. Annu. Rev. Med. 55, 61–95 (2004).
Kurreck, J. Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem. 270, 1628–1644 (2003).
Yu, R. Z. et al. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 35, 460–468 (2007).
Crooke, R. M. et al. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46, 872–884 (2005).
Merki, E. et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation 118, 743–753 (2008).
Yu, R. Z. et al. Cross-species comparison of in vivo PK/PD relationships for second-generation antisense oligonucleotides targeting apolipoprotein B-100. Biochem. Pharmacol. 77, 910–919 (2009).
Kastelein, J. J. et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 114, 1729–1735 (2006).
Yu, R. Z. et al. Lack of pharmacokinetic interaction of mipomersen sodium (ISIS 301012), a 2′-O-methoxyethyl modified antisense oligonucleotide targeting apolipoprotein B-100 messenger RNA, with simvastatin and ezetimibe. Clin. Pharmacokinet. 48, 39–50 (2009).
Akdim, F. et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J. Am. Coll. Cardiol. 55, 1611–1618 (2010).
Rader, D. J., Cohen, J. & Hobbs, H. H. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J. Clin. Invest. 111, 1795–1803 (2003).
Akdim, F. et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am. J. Cardiol. 105, 1413–1419 (2010).
Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010).
Kane, J. P. & Havel, R. J. in Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R., Sly, W. S. & Childs, B.) Ch. 115 (McGraw-Hill, New York, 2001) [online], (2001).
Fazio, S. & Linton, M. F. in Clinical Lipidology: a Companion to Braunwald's Heart Disease (ed. Ballantyne, C. M.) 11–25 (Saunders, Philadelphia, 2008).
Wetterau, J. R. et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 (1992).
Sharp, D. et al. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinemia. Nature 365, 65–69 (1993).
Rader, D. J. & Brewer, H. B. Jr. Abetalipoproteinemia: new insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease. JAMA 270, 865–869 (1993).
Wetterau, J. R. et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science 282, 751–754 (1998).
Liao, W., Hui, T. Y., Young, S. G. & Davis, R. A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J. Lipid Res. 44, 978–985 (2003).
Cuchel, M. et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356, 148–156 (2007).
Samaha, F. F., McKenney, J., Bloedon, L. T., Sasiela, W. J. & Rader, D. J. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 5, 497–505 (2008).
Cohen, J. et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 (2005).
Berge, K. E., Ose, L. & Leren, T. P. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 26, 1094–1100 (2006).
Kotowski, I. K. et al. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78, 410–422 (2006).
Zhao, Z. et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79, 514–523 (2006).
Dubuc, G. et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24, 1454–1459 (2004).
Dubuc, G. et al. A new method for measurement of total plasma PCSK9: clinical applications. J. Lipid Res. 51, 140–149 (2010).
Chan, J. C. et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl Acad. Sci. USA 106, 9820–9825 (2009).
Regeneron. Clinical pipeline [online], (2010).
Abifadel, M. et al. Strategies for proprotein convertase subtilisin kexin 9 modulation: a perspective on recent patents. Expert Opin. Ther. Pat. 20, 1547–1571 (2010).
Ni, Y. G. et al. A proprotein convertase subtilisin-like/kexin type 9 (PCSK9)-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces free circulating PCSK9 and LDL-cholesterol. J. Lipid Res. 52, 78–86 (2011).
Graham, M. J. et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J. Lipid Res. 48, 763–767 (2007).
Jepsen, J. S., Sørensen, M. D. & Wengel, J. Locked nucleic acid: a potent nucleic acid analog in therapeutics and biotechnology. Oligonucleotides 14, 130–146 (2004).
Gupta, N. et al. A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS One 5, e10682 (2010).
Santaris Pharma A/S. Santaris Pharma A/S advances RNA-targeted drug development candidate against PCSK9, an important new target for the treatment of high cholesterol [online], (2010).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Frank-Kamenetsky, M. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl Acad. Sci. USA 105, 11915–11920 (2008).
Alnylam Pharmaceuticals. Hypercholesterolemia [online], (2008).
Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA 231, 360–381 (1975).
Angelin, B. & Rudling, M. Lipid lowering with thyroid hormone and thyromimetics. Curr. Opin. Lipidol. 21, 499–506 (2010).
Baxter, J. D. & Webb, P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 8, 308–320 (2009).
Ladenson, P. W. et al. Use of the thyroid hormone analog eprotirome in statin-treated dyslipidemia. N. Engl. J. Med. 362, 906–916 (2010).
Brown, M. S. & Goldstein, J. L. A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 (1986).
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Li, J. et al. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem. J. 406, 203–207 (2007).
Maxwell, K. N. & Breslow, J. L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl Acad. Sci. USA. 101, 7100–7105 (2004).
Regeneron. Regeneron provides initial data on two antibody product candidates [online], (2010).
Luo, Y. et al. Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J. Lipid Res. 50, 1581–1588 (2009).
Steinberg, D. & Witztum, J. L. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc. Natl Acad. Sci. USA 106, 9, 546–9, 547 (2009).
ISIS Pharmaceuticals. Drugs in development [online], (2010).
U. S. NIH. Safety study of BMS-844421 for treatment of hypercholesterolemia [online], (2011).
Adnexus. Our science. Adnectin product class [online], (2008).
Author information
Authors and Affiliations
Contributions
C. M. Ballantyne and A. Brautbar researched data for the article, discussed the content, wrote the article, and reviewed/edited the manuscript before submission and after peer-review.
Corresponding author
Ethics declarations
Competing interests
C. M. Ballantyne has received grants/research support from Abbott, AHA, American Diabetes Association, AstraZeneca, GlaxoSmithKline, Merck & Co., NIH, Roche, Sanofi-Synthelabo, and Takeda. He has acted as a consultant for Abbott, Adnexus, Amylin, Bristol-Myers Squibb, Genentech, Idera Pharma, Kowa, Merck & Co., NicOx, Novartis, Resverlogix, Roche, Sanofi-Synthelabo, and Takeda. He has received speakers' bureau honoraria from Abbott, AstraZeneca, GlaxoSmithKline, and Merck & Co. A. Brautbar declares no competing interests.
Rights and permissions
About this article
Cite this article
Brautbar, A., Ballantyne, C. Pharmacological strategies for lowering LDL cholesterol: statins and beyond. Nat Rev Cardiol 8, 253–265 (2011). https://doi.org/10.1038/nrcardio.2011.2
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.2
This article is cited by
-
Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology
Nature Mental Health (2024)
-
Targeting the cholesterol-RORα/γ axis inhibits colorectal cancer progression through degrading c-myc
Oncogene (2022)
-
AMPK activator C24 inhibits hepatic lipogenesis and ameliorates dyslipidemia in HFHC diet-induced animal models
Acta Pharmacologica Sinica (2021)
-
Besnoitia besnoiti infection alters both endogenous cholesterol de novo synthesis and exogenous LDL uptake in host endothelial cells
Scientific Reports (2019)
-
SLC12A3 variants modulate LDL cholesterol levels in the Mongolian population
Lipids in Health and Disease (2017)