Abstract
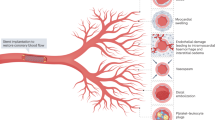
Since early reperfusion therapy for patients with acute myocardial infarction (AMI) was demonstrated to decrease mortality, numerous improvements in AMI management have focused on prompt reperfusion of the epicardial coronary arteries. However, in a substantial group of patients with AMI, reperfusion of the myocardial tissue is hindered by dysfunction of the microvasculature, despite successful restoration of the epicardial coronary flow. These patients have prolonged ischemia and an adverse clinical outcome. Although several studies investigating the etiology of microvascular dysfunction have been performed, little is known about the restoration process of microvascular dysfunction after reperfused AMI. The objective of this Review is to summarize our knowledge on natural restoration of the microvasculature after reperfused AMI, particularly with regard to angiogenesis, discuss diagnostic modalities used to identify patients with microvascular dysfunction and highlight the potential of pharmacological and cellular interventions to stimulate the recovery of the microvasculature by promoting angiogenesis.
Key Points
-
Despite successful primary percutaneous coronary intervention, a large proportion of patients with acute myocardial infarction show inadequate myocardial perfusion, owing to dysfunction of the microcirculation
-
Microvascular dysfunction is associated with an adverse clinical outcome
-
Tissue edema, vasoconstriction, endothelial cell swelling, inflammation, neutrophil plugging and the embolization of atheromatous and thrombotic debris are all causes of microvascular dysfunction
-
Damaged microvasculature can be restored by angiogenesis
-
Several growth factors and circulating mononuclear cells, including bone marrow mononuclear cells, are involved in the process of angiogenesis
-
Targeting angiogenesis constitutes a potential new treatment modality to counteract the negative effects of microvascular dysfunction after mechanically reperfused myocardial infarction
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Keeley, E. C., Boura, J. A. & Grines, C. L. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 367, 579–588 (2006).
Brodie, B. R. et al. Importance of infarct-related artery patency for recovery of left ventricular function and late survival after primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 28, 319–325 (1996).
Brodie, B. R. et al. Importance of infarct-related artery patency for recovery of left ventricular function and late survival after primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 28, 319–325 (1996).
Grines, C. L. et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N. Engl. J. Med. 328, 673–679 (1993).
Kennedy, J. W. et al. The western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction. A 12-month follow-up report. N. Engl. J. Med. 312, 1073–1078 (1985).
Wu, K. C. et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97, 765–772 (1998).
Koch, K. T. et al. Proximal embolic protection with aspiration in percutaneous coronary intervention using the Proxis device. Rev. Cardiovasc. Med. 8, 160–166 (2007).
Sardella, G. et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J. Am. Coll. Cardiol. 53, 309–315 (2009).
Vlaar, P. J. et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371, 1915–1920 (2008).
Petronio, A. S. et al. Left ventricular remodeling after primary coronary angioplasty in patients treated with abciximab or intracoronary adenosine. Am. Heart J. 150, 1015 (2005).
Kunadian, V. et al. Intracoronary pharmacotherapy in the management of coronary microvascular dysfunction. J. Thromb. Thrombolysis 26, 234–242 (2008).
Ramaraj, R. & Movahed, M. R. Microvascular dysfunction following primary percutaneous coronary intervention in the setting of ST-elevation myocardial infarction. J. Invasive Cardiol. 20, 603–614 (2008).
Srinivasan, M., Rihal, C., Holmes, D. R. & Prasad, A. Adjunctive thrombectomy and distal protection in primary percutaneous coronary intervention: impact on microvascular perfusion and outcomes. Circulation 119, 1311–1319 (2009).
Bonderman, D. et al. Coronary no-reflow is caused by shedding of active tissue factor from dissected atherosclerotic plaque. Blood 99, 2794–2800 (2002).
Hirata, K., Matsuda, Y., Akita, H., Yokoyama, M. & Fukuzaki, H. Myocardial ischaemia induced by endothelin in the intact rabbit: angiographic analysis. Cardiovasc. Res. 24, 879–883 (1990).
Ma, X. L., Weyrich, A. S., Lefer, D. J. & Lefer, A. M. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ. Res. 72, 403–412 (1993).
Maxwell, L. & Gavin, J. Anti-oxidant therapy improves microvascular ultrastructure and perfusion in postischemic myocardium. Microvasc. Res. 43, 255–266 (1992).
Maier, W. et al. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation 111, 1355–1361 (2005).
Sheridan, F. M., Cole, P. G. & Ramage, D. Leukocyte adhesion to the coronary microvasculature during ischemia and reperfusion in an in vivo canine model. Circulation 93, 1784–1787 (1996).
Kotani, J. et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation 106, 1672–1677 (2002).
Manciet, L. H., Poole, D. C., McDonagh, P. F., Copeland, J. G. & Mathieu-Costello, O. Microvascular compression during myocardial ischemia: mechanistic basis for no-reflow phenomenon. Am. J. Physiol. 266, H1541–H1550 (1994).
Maxwell, L. & Gavin, J. B. The role of post-ischaemic reperfusion in the development of microvascular incompetence and ultrastructural damage in the myocardium. Basic Res. Cardiol. 86, 544–553 (1991).
Matsumura, K., Jeremy, R. W., Schaper, J. & Becker, L. C. Progression of myocardial necrosis during reperfusion of ischemic myocardium. Circulation 97, 795–804 (1998).
Tonnesen, M. G., Feng, X. & Clark, R. A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5, 40–46 (2000).
Conway, E. M., Collen, D. & Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 49, 507–521 (2001).
Kim, S. J. et al. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am. J. Pathol. 174, 1972–1980 (2009).
Kamei, M. et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 (2006).
Gray, C. et al. Ischemia is not required for arteriogenesis in zebrafish embryos. Arterioscler. Thromb. Vasc. Biol. 27, 2135–2141 (2007).
van Royen N. et al. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc. Res. 49, 543–553 (2001).
Pipp, F. et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler. Thromb. Vasc. Biol. 24, 1664–1668 (2004).
Simons, M. et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation 102, E73–E86 (2000).
Henriques, J. P. et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 23, 1112–1117 (2002).
Sezer, M. et al. Relationship between collateral blood flow and microvascular perfusion after reperfused acute myocardial infarction. Jpn. Heart J. 44, 855–863 (2003).
Frangogiannis, N. G., Michael, L. H. & Entman, M. L. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc. Res. 48, 89–100 (2000).
Ren, G., Michael, L. H., Entman, M. L. & Frangogiannis, N. G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J. Histochem. Cytochem. 50, 71–79 (2002).
Arras, M. et al. Tumor necrosis factor-alpha is expressed by monocytes/macrophages following cardiac microembolization and is antagonized by cyclosporine. Basic Res. Cardiol. 93, 97–107 (1998).
Lee, S. H. et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 342, 626–633 (2000).
Frangogiannis, N. G. et al. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 15, 1428–1430 (2001).
Zachary, I. & Gliki, G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc. Res. 49, 568–581 (2001).
Weis, S. et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Clin. Invest. 113, 885–894 (2004).
Banai, S. et al. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc. Res. 28, 1176–1179 (1994).
Kranz, A., Rau, C., Kochs, M. & Waltenberger, J. Elevation of vascular endothelial growth factor-A serum levels following acute myocardial infarction. Evidence for its origin and functional significance. J. Mol. Cell. Cardiol. 32, 65–72 (2000).
Soeki, T. et al. Serial changes in serum VEGF and HGF in patients with acute myocardial infarction. Cardiology 93, 168–174 (2000).
Pannitteri, G., Petrucci, E. & Testa, U. Coordinate release of angiogenic growth factors after acute myocardial infarction: evidence of a two-wave production. J. Cardiovasc. Med. (Hagerstown) 7, 872–879 (2006).
Saeed, M. et al. Adeno-associated viral vector-encoding vascular endothelial growth factor gene: effect on cardiovascular MR perfusion and infarct resorption measurements in swine. Radiology 243, 451–460 (2007).
Koneru, S. et al. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J. Cell. Mol. Med. 12, 2651–2664 (2008).
Jones, N., Iljin, K., Dumont, D. J. & Alitalo, K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2, 257–267 (2001).
Oike, Y., Yasunaga, K. & Suda, T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int. J. Hematol. 80, 21–28 (2004).
Shyu, K. G., Chang, C. C., Wang, B. W., Kuan, P. & Chang, H. Increased expression of angiopoietin-2 and Tie2 receptor in a rat model of myocardial ischaemia/reperfusion. Clin. Sci. (Lond.) 105, 287–294 (2003).
Xaymardan, M. et al. Senescent impairment in synergistic cytokine pathways that provide rapid cardioprotection in the rat heart. J. Exp. Med. 199, 797–804 (2004).
Carmeliet, P. Fibroblast growth factor-1 stimulates branching and survival of myocardial arteries: a goal for therapeutic angiogenesis? Circ. Res. 87, 176–178 (2000).
Wang, Y. et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart 92, 768–774 (2006).
Waltenberger, J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation 96, 4083–4094 (1997).
Battler, A. et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J. Am. Coll. Cardiol. 22, 2001–2006 (1993).
Horrigan, M. C. et al. Reduction in myocardial infarct size by basic fibroblast growth factor after temporary coronary occlusion in a canine model. Circulation 94, 1927–1933 (1996).
Scheinowitz, M. et al. Effect of basic fibroblast growth factor on left ventricular geometry in rats subjected to coronary occlusion and reperfusion. Isr. Med. Assoc. J. 4, 109–113 (2002).
Fukuyama, N. et al. Intravenous injection of phagocytes transfected ex vivo with FGF4 DNA/biodegradable gelatin complex promotes angiogenesis in a rat myocardial ischemia/reperfusion injury model. Basic Res. Cardiol. 102, 209–216 (2007).
Naldini, L. et al. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene 6, 501–504 (1991).
Bhargava, M. et al. Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ. 3, 11–20 (1992).
Ono, K., Matsumori, A., Shioi, T., Furukawa, Y. & Sasayama, S. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation 95, 2552–2558 (1997).
Yasuda, S. et al. Enhanced secretion of cardiac hepatocyte growth factor from an infarct region is associated with less severe ventricular enlargement and improved cardiac function. J. Am. Coll. Cardiol. 36, 115–121 (2000).
Chen, X. H. et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia–reperfusion injury through its multiple actions. J. Card. Fail. 13, 874–883 (2007).
Saeed, M. et al. MR assessment of myocardial perfusion, viability, and function after intramyocardial transfer of VM202, a new plasmid human hepatocyte growth factor in ischemic swine myocardium. Radiology 249, 107–118 (2008).
Birdsall, H. H. et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation 95, 684–692 (1997).
Dimmeler, S., Burchfield, J. & Zeiher, A. M. Cell-based therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 28, 208–216 (2008).
Massa, M. et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 105, 199–206 (2005).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Quaini, F. et al. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Gnecchi, M., Zhang, Z., Ni, A. & Dzau, V. J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219 (2008).
Xu, M. et al. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J. Mol. Cell. Cardiol. 42, 441–448 (2007).
Halkos, M. E. et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res. Cardiol. 103, 525–536 (2008).
Schuleri, K. H. et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am. J. Physiol. Heart Circ. Physiol. 294, H2002–H2011 (2008).
Tang, J., Xie, Q., Pan, G., Wang, J. & Wang, M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur. J. Cardiothorac. Surg. 30, 353–361 (2006).
Templin, C. et al. Ex vivo expanded hematopoietic progenitor cells improve cardiac function after myocardial infarction: role of beta-catenin transduction and cell dose. J. Mol. Cell. Cardiol. 45, 394–403 (2008).
Kanellakis, P., Slater, N. J., Du, X. J., Bobik, A. & Curtis, D. J. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc. Res. 70, 117–125 (2006).
Sato, T. et al. G-CSF after myocardial infarction accelerates angiogenesis and reduces fibrosis in swine. Int. J. Cardiol. 127, 166–173 (2008).
Kern, M. J. et al. Determination of angiographic (TIMI grade) blood flow by intracoronary Doppler flow velocity during acute myocardial infarction. Circulation 94, 1545–1552 (1996).
Henriques, J. P. et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107, 2115–2119 (2003).
Iwakura, K. et al. Alternation in the coronary blood flow velocity pattern in patients with no reflow and reperfused acute myocardial infarction. Circulation 94, 1269–1275 (1996).
Montisci, R. et al. Non-invasive coronary flow reserve is correlated with microvascular integrity and myocardial viability after primary angioplasty in acute myocardial infarction. Heart 92, 1113–1118 (2006).
Bax, M. et al. Short- and long-term recovery of left ventricular function predicted at the time of primary percutaneous coronary intervention in anterior myocardial infarction. J. Am. Coll. Cardiol. 43, 534–541 (2004).
Wu, K. C. et al. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J. Am. Coll. Cardiol. 32, 1756–1764 (1998).
Okamura, A. et al. Usefulness of a new grading system based on coronary flow velocity pattern in predicting outcome in patients with acute myocardial infarction having percutaneous coronary intervention. Am. J. Cardiol. 96, 927–932 (2005).
Kim, R. J., Chen, E. L., Lima, J. A. & Judd, R. M. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94, 3318–3326 (1996).
Hirsch, A. et al. Relation between the assessment of microvascular injury by cardiovascular magnetic resonance and coronary Doppler flow velocity measurements in patients with acute anterior wall myocardial infarction. J. Am. Coll. Cardiol. 51, 2230–2238 (2008).
Nijveldt, R. et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J. Am. Coll. Cardiol. 52, 181–189 (2008).
Yla-Herttuala, S., Markkanen, J. E. & Rissanen, T. T. Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc. Med. 14, 295–300 (2004).
Takano, H. et al. Feasibility and safety of granulocyte colony-stimulating factor treatment in patients with acute myocardial infarction. Int. J. Cardiol. 122, 41–47 (2007).
Valgimigli, M. et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur. Heart J. 26, 1838–1845 (2005).
Kang, H. J. et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 363, 751–756 (2004).
Assmus, B. et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 106, 3009–3017 (2002).
Strauer, B. E. et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 (2002).
Erbs, S. et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation 116, 366–374 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
van der Laan, A., Piek, J. & van Royen, N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol 6, 515–523 (2009). https://doi.org/10.1038/nrcardio.2009.103
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.103
This article is cited by
-
ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis
Biology Direct (2023)
-
Combination human umbilical cord perivascular and endothelial colony forming cell therapy for ischemic cardiac injury
npj Regenerative Medicine (2023)
-
[68Ga]Ga-NODAGA-E[(cRGDyK)]2 angiogenesis PET following myocardial infarction in an experimental rat model predicts cardiac functional parameters and development of heart failure
Journal of Nuclear Cardiology (2023)
-
Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction
Basic Research in Cardiology (2023)
-
Myocardial angiogenesis induced by concurrent vitamin D supplementation and aerobic-resistance training is mediated by inhibiting miRNA-15a, and miRNA-146a and upregulating VEGF/PI3K/eNOS signaling pathway
Pflügers Archiv - European Journal of Physiology (2023)