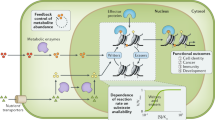
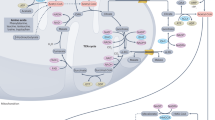
Abstract
It is well known that an insufficiency of dietary methyl-group donors can cause cancer, and that a deficiency in methylation is characteristic of cancer, but how carcinogenesis results from abnormal methyl-donor metabolism has long remained a matter of speculation. Recently, however, it has been found that some histone methyltransferases, which require methyl donors for activity, are tumour suppressors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
17 May 2002
There was an error in the third paragraph, fourth sentance: this originally read "More recently, it was realized that they belong to a protein superfamily that contains one of two similar... "They belong to" and "that" were removed and the sentance re-written by request of the author. This incorrect version appeared as CP onlin material for c. one week.
Notes
There was an error in the third paragraph of the advanced online publication of this article. The error has now been corrected in the HTML and PDF versions, and the article is correct in the print issue.
References
Doll, R. & Peto, R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl Cancer Inst. 66, 1191–1308 (1981).
Feinberg, A. P. & Vogelstein, B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92 (1983).
Baylin, S. B. & Herman, J. G. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 16, 168–174 (2000).
Jones, P. A. & Laird, P. W. Cancer epigenetics comes of age. Nature Genet. 21, 163–167 (1999).
Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074–1080 (2001).
Jiang, G.-L. & Huang, S. The yin–yang of PR-domain family genes in tumorigenesis. Histol. Histopathol. 15, 109–117 (2000).
Tschiersch, B. et al. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 13, 3822–3831 (1994).
Jones, R. S. & Gelbart, W. M. The Drosophila Polycomb-group gene Enhancer of zeste contains a region with sequence similarity to trithorax. Mol. Cell. Biol. 13, 6357–6366 (1993).
Buyse, I. M., Shao, G. & Huang, S. The retinoblastoma protein binds to RIZ, a zinc finger protein that shares an epitope with the adenovirus E1A protein. Proc. Natl Acad. Sci. USA 92, 4467–4471 (1995).
Huang, S. Blimp-1 is the murine homolog of the human transcriptional repressor PRDI–BF1. Cell 78, 9 (1994).
Huang, S., Shao, G. & Liu, L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J. Biol. Chem. 273, 15933–15940 (1998).
Cui, X. et al. Association of SET domain and myotubularin-related proteins modulates growth control. Nature Genet. 18, 331–337 (1998).
Cardoso, C. et al. Specific interaction between the XNP/ATR-X gene product and the SET domain of the human EZH2 protein. Hum. Mol. Genet. 7, 679–684 (1998).
Rozenblatt-Rosen, O. et al. The C-terminal SET domains of ALL-1 and TRITHORAX interact with the INI1 and SNR1 proteins, components of the SWI/SNF complex. Proc. Natl Acad. Sci. USA 95, 4152–4157 (1998).
Rea, S. et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599 (2000).
Fears, S. et al. Intergenic splicing of MDS1 and EVII occurs in normal tissues as well as in myeloid leukemia and produces a new member of the PR domain family. Proc. Natl Acad. Sci. USA 93, 1642–1647 (1996).
Steele-Perkins, G. et al. Tumor formation and inactivation of RIZ1, an Rb-binding member of a nuclear protein-methyltransferase superfamily. Genes Dev. 15, 2250–2262 (2001).
Liu, L., Shao, G., Steele-Perkins, G. & Huang, S. The retinoblastoma interacting zinc finger gene RIZ produces a PR domain lacking product through an internal promoter. J. Biol. Chem. 272, 2984–2991 (1997).
Buyse, I. M., Takahashi, E. & Huang, S. Physical mapping of the retinoblastoma-interacting zinc finger gene RIZ to D1S228 on chromosome 1p36. Genomics 34, 119–121 (1996).
Chadwick, R. B. et al. Candidate tumor suppressor RIZ is frequently involved in colorectal carcinogenesis. Proc. Natl Acad. Sci. USA 97, 2662–2667 (2000).
He, L. et al. RIZ1, but not the alternative RIZ2 product of the same gene, is underexpressed in breast cancer, and forced RIZ1 expression causes G2-M cell cycle arrest and/or apoptosis. Cancer Res. 58, 4238–4244 (1998).
Jiang, G.-L., Liu, L., Buyse, I. M., Simon, D. & Huang, S. Decreased RIZ1 expression but not RIZ2 in hepatoma and suppression of hepatoma tumorigenicity by RIZ1. Int. J. Cancer 83, 541–547 (1999).
Du, Y. et al. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res. 61, 8094–8099 (2001).
Jiang, G. L. & Huang, S. Adenovirus expressing RIZ1 in tumor suppressor gene therapy of microsatellite-unstable colorectal cancers. Cancer Res. 61, 1796–1798 (2001).
Nucifora, G. et al. Consistent intergenic splicing and production of multiple transcripts between AML1 at 21q22 and unrelated genes at 3q26 in (3;21)(q26;q22) translocations. Proc. Natl Acad. Sci. USA 91, 4004–4008 (1994).
Morishita, K. et al. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell 54, 831–840 (1988).
Gu, Y. et al. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL1 gene, related to Drosophila trithorax, to the AF4 gene. Cell 71, 701–708 (1992).
Tkachuk, D. C., Kohler, S. & Cleary, M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell 71, 691–700 (1992).
Djabali, M. et al. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nature Genet. 2, 113–118 (1992).
Arakawa, H. et al. Identification and characterization of the ARP1 gene, a target for the human acute leukemia ALL1 gene. Proc. Natl Acad. Sci. USA 95, 4573–4578 (1998).
Baffa, R., Negrini, M., Schichman, S. A., Huebner, K. & Croce, C. M. Involvement of the ALL1 gene in a solid tumor. Proc. Natl Acad. Sci. USA 92, 4922–4926 (1995).
Peters, A. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Nielsen, S. J. et al. Rb targets histone H3 methylation and HP1 to promoters. Nature 412, 561–565 (2001).
Bannister, A. J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromodomain. Nature 410, 120–124 (2001).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001).
Kurotaki, N. et al. Haploinsufficiency of NSD1 causes Sotos syndrome. Nature Genet. 30, 365–366 (2002).
Chesi, M. et al. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood 92, 3025–3034 (1998).
Lin, Y., Wong, K.-K. & Calame, K. Repression of c-Myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276, 596–598 (1997).
Mochizuki, N. et al. A novel gene, MEL1, mapped to 1p36.3 is highly homologous to the MDS1/EVI1 gene and is transcriptionally activated in t(1;3)(p36;q21)-positive leukemia cells. Blood 96, 3209–3214 (2000).
Caudill, M. A. et al. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine β-synthase heterozygous mice. J Nutr 131, 2811–2818 (2001).
Hershfield, M. S. & Krodich, N. M. S-adenosyl-homocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science 202, 757–760 (1978).
Williams-Ashman, H. G., Seidenfeld, J. & Galletti, P. Trends in the biochemical pharmacology of 5′-deoxy-5′-methylthioadenosine. Biochem. Pharmacol. 31, 277–288 (1982).
Mikol, Y. B., Hoover, K. L., Creasia, D. & Poirier, L. A. Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis 4, 1619–1629 (1983).
Ghoshal, A. K. & Farber, E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis 5, 1367–1370 (1984).
Shivapurkar, N. & Poirier, L. A. Tissue levels of S-adenosyl-methionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis 4, 1051–1057 (1983).
Cravo, M. L. et al. Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine-treated rats. Cancer Res. 52, 5002–5006 (1992).
Christensen, B. et al. Correlation of a common mutation in the methylenetetrahydrofolate reductase gene with plasma homocysteine in patients with premature coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 17, 569–573 (1997).
Fullerton, F. R., Hoover, K., Mikol, Y. B., Creasia, D. A. & Poirier, L. A. The inhibition by methionine and choline of liver carcinoma formation in male C3H mice dosed with diethylnitrosamine and fed phenobarbital. Carcinogenesis 11, 1301–1305 (1990).
Giovannucci, E. et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann. Intern. Med. 129, 517–524 (1998).
Prinz-Langenohl, R., Fohr, I. & Pietrzik, K. Beneficial role for folate in the prevention of colorectal and breast cancer. Eur. J. Nutrit. 40, 98–105 (2001).
Butterworth, C. E. Jr, Hatch, K. D., Gore, H., Mueller, H. & Krumdieck, C. L. Improvement in cervical dysplasia associated with folic acid therapy in users of oral contraceptives. Am. J. Clin. Nutr. 35, 73–82 (1982).
Heimburger, D. C. et al. Improvement in bronchial squamous metaplasia in smokers treated with folate and vitamin B12. Report of a preliminary randomized, double-blind intervention trial. JAMA 259, 1525–1530 (1988).
Hartman, T. J. et al. Association of the B-vitamins pyridoxal 5′-phosphate (B(6)), B(12), and folate with lung cancer risk in older men. Am. J. Epidemiol. 153, 688–694 (2001).
Kato, I. et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case–control study. Br. J. Cancer 79, 1917–1922 (1999).
Chello, P. L. & Bertino, J. R. Dependence of 5-methyltetra-hydrofolate utilization by L5178Y murine leukemia cells in vitro on the presence of hydroxycobalamin and transcobalamin II. Cancer Res. 33, 1898–1904 (1973).
Hoffman, R. M. & Erbe, R. W. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc. Natl Acad. Sci. USA 73, 1523–1527 (1976).
Stern, P. H. & Hoffman, R. M. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro 20, 663–670 (1984).
Toohey, J. I. Methylthio group cleavage from methylthioadenosine. Description of an enzyme and its relationship to the methylthio requirement of certain cells in culture. Biochem. Biophys. Res. Commun. 78, 1273–1280 (1977).
Nobori, T. et al. Genomic cloning of methylthioadenosine phosphorylase: a purine metabolic enzyme deficient in multiple different cancers. Proc. Natl Acad. Sci. USA 93, 6203–6208 (1996).
Tang, B. Defects in methylthioadenosine phosphorylase are associated with but not responsible for methionine-dependent tumor cell growth. Cancer Res. 60, 5543–5547 (2000).
Dreyling, M. H., Roulston, D., Bohlander, S. K., Vardiman, J. & Olopade, O. I. Codeletion of CDKN2 and MTAP genes in a subset of non-Hodgkin's lymphoma may be associated with histologic transformation from low-grade to diffuse large-cell lymphoma. Genes Chromosom. Cancer 22, 72–78 (1998).
Schmid, M. et al. Homozygous deletions of methylthioadenosine phosphorylase (MTAP) are more frequent than p16INK4A (CDKN2) homozygous deletions in primary non-small cell lung cancers (NSCLC). Oncogene 17, 2669–2675 (1998).
Pegg, A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 48, 759–774 (1988).
Megosh, L. et al. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res. 55, 4205–4209 (1995).
Redman, C. et al. Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells. Carcinogenesis 18, 1195–1202 (1997).
Wainfan, E. & Poirier, L. A. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res. 52, 2071s–2077s (1992). | PubMed |
Rushmore, T. H. et al. A choline-devoid diet, carcinogenic in the rat, induces DNA damage and repair. Carcinogenesis 7, 1677–1680 (1986).
James, S. J., Miller, B. J., Cross, D. R., McGarrity, L. J. & Morris, S. M. The essentiality of folate for the maintenance of deoxynucleotide precursor pools, DNA synthesis, and cell cycle progression in PHA-stimulated lymphocytes. Environ. Health Perspect. 101 (Suppl. 5), 173–178 (1993).
da Costa, K. A., Cochary, E. F., Blusztajn, J. K., Garner, S. C. & Zeisel, S. H. Accumulation of 1,2-sn-diradylglycerol with increased membrane-associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J. Biol. Chem. 268, 2100–2105 (1993).
Laird, P. W. et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell 81, 197–205 (1995).
Blount, B. C. et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl Acad. Sci. USA 94, 3290–3295 (1997).
Duthie, S. J., Grant, G. & Narayanan, S. Increased uracil misincorporation in lymphocytes from folate-deficient rats. Br. J. Cancer 83, 1532–1537 (2000).
Houlston, R. S. & Tomlinson, I. P. Polymorphisms and colorectal tumor risk. Gastroenterology 121, 282–301 (2001).
Ma, J. et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 57, 1098–1102 (1997).
Sohn, K. J. et al. The effect of dietary folate on Apc and p53 mutations in the dimethylhydrazine rat model of colorectal cancer. Carcinogenesis 20, 2345–2350 (1999).
Corda, Y. et al. Interaction between Set1p and checkpoint protein Mec3p in DNA repair and telomere functions. Nature Genet. 21, 204–208 (1999).
McCully, K. S. Homocystinuria, arteriosclerosis, methylmalonic aciduria, and methyltransferase deficiency: a key case revisited. Nutr. Rev. 50, 7–12 (1992).
Seshadri, S. et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N. Engl. J. Med. 346, 476–483 (2002).
Tsai, J. C. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc. Natl Acad. Sci. USA 91, 6369–6373 (1994).
Gottlieb, P. D. et al. BOP encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nature Genet. 1 April 2002 (DOI:10.1038/ng866).
Brattstrom, L., Lindgren, A., Israelsson, B., Andersson, A. & Hultberg, B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J. Internal Med. 236, 633–641 (1994).
Giovannucci, E. et al. Alcohol, low-methionine–low-folate diets, and risk of colon cancer in men. J. Natl Cancer Inst. 87, 265–273 (1995).
Wu, K. et al. A prospective study on folate, B12, and pyridoxal 5′-phosphate (B6) and breast cancer. Cancer Epidemiol Biomarkers Prev 8, 209–217 (1999).
Hsing, A. W. et al. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 71, 745–750 (1993).
Stolzenberg-Solomon, R. Z. et al. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J. Natl Cancer Inst. 91, 535–541 (1999).
Esteller, M., Garcia, A., Martinez-Palones, J. M., Xercavins, J. & Reventos, J. Germ line polymorphisms in cytochrome-P450 1A1 (C4887 CYP1A1) and methylenetetrahydrofolate reductase (MTHFR) genes and endometrial cancer susceptibility. Carcinogenesis 18, 2307–2311 (1997).
Matsuo, K. et al. Association between polymorphisms of folate- and methionine-metabolizing enzymes and susceptibility to malignant lymphoma. Blood 97, 3205–3209 (2001).
Gershoni-Baruch, R. et al. Association of the C677T polymorphism in the MTHFR gene with breast and/or ovarian cancer risk in Jewish women. Eur. J. Cancer 36, 2313–2316 (2000).
Piyathilake, C. J. et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism increases the risk of cervical intraepithelial neoplasia. Anticancer Res. 20, 1751–1757 (2000).
Shen, H. et al. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of gastric cancer in a Chinese population: a case–control study. Int. J. Cancer 95, 332–336 (2001).
Ma, J. et al. A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 8, 825–829 (1999).
Author information
Authors and Affiliations
Supplementary information
Related links
Related links
DATABASES
Cancer.gov
LocusLink
OMIM
<i>Saccharomyces</i> Genome Database
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Huang, S. Histone methyltransferases, diet nutrients and tumour suppressors. Nat Rev Cancer 2, 469–476 (2002). https://doi.org/10.1038/nrc819
Issue Date:
DOI: https://doi.org/10.1038/nrc819
This article is cited by
-
Activity of pemetrexed in pre-clinical chordoma models and humans
Scientific Reports (2023)
-
NvPrdm14d-expressing neural progenitor cells contribute to non-ectodermal neurogenesis in Nematostella vectensis
Nature Communications (2023)
-
Current basis and future directions of zebrafish nutrigenomics
Genes & Nutrition (2019)
-
Colibactin assembly line enzymes use S-adenosylmethionine to build a cyclopropane ring
Nature Chemical Biology (2017)
-
Sequences analyses and expression profiles in tissues and embryos of Japanese flounder (Paralichthys olivaceus) PRDM1
Fish Physiology and Biochemistry (2016)