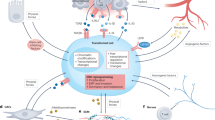
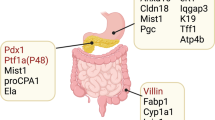
Key Points
-
Sporadic cancer development is triggered by initiating mutations in a single cell, which result in tumour growth in a genetically wild-type environment that might actively contribute to tumour progression.
-
Tumour models that use conventional transgenic or knockout mice do not allow modelling of sporadic cancer, because the initiating mutation is present in all cells of a specific tissue or throughout the body. These cells include those that constitute the tumour microenvironment.
-
Various methods for conditional tumour-suppressor gene mutation and oncogene activation allow modelling of sporadic cancer in genetically engineered mice.
-
Recombinase-mediated conditional gene mutation can be used successfully to circumvent embryonic lethality or unwanted tumorigenesis in conventional tumour-suppressor gene knockouts.
-
Tumour growth and maintenance in mice with regulatable oncogene expression depends, in most cases, on continuous oncogene expression, supporting the validity of these factors as therapeutic targets.
-
Combining mouse models of sporadic cancer with various genome-wide screens for genetic alterations allows a comparison, on a molecular level, of mouse tumours with their human counterparts, and facilitates the discovery of new cancer genes and pathways that are involved in multistep tumorigenesis.
-
Mouse imaging systems that enable the non-invasive monitoring of tumour development will render conditional mouse models of sporadic cancer particularly useful for preclinical testing of therapeutic intervention or chemoprevention strategies.
Abstract
First-generation mouse tumour models, which used transgenic mice or conventional knockouts, are now being superseded by models that are based on conditional knockouts and mice that carry regulatable oncogenes. In these mice, somatic mutations can be induced in a tissue-specific and time-controlled fashion, which more faithfully mimics sporadic tumour formation. These second-generation models provide exciting new opportunities to gain insight into the contribution of known and unknown genes in the initiation, progression and treatment of cancer, and mimic human cancer better than ever before.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morse, H. C. Origins of Inbred Mice (Academic Press, New York, 1978).
Furth, J., Seibold, H. R. & Rathbone, R. R. Experimental studies on lymphomatosis of mice. Am. J. Cancer 19, 521–526 (1933).
Hilgers, J. & Sluyser, M. Mammary Tumors in the Mouse (Elsevier/North-Holland Biomedical Press, Amsterdam, 1981).
Bittner, J. J. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science 84, 162 (1936).
Li, J. et al. Leukaemia disease genes: large-scale cloning and pathway predictions. Nature Genet. 23, 348–353 (1999).
Moser, A. R., Pitot, H. C. & Dove, W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Su, L. K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Brinster, R. L. et al. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell 37, 367–379 (1984).
Stewart, T. A., Pattengale, P. K. & Leder, P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38, 627–637 (1984).
Robertson, E., Bradley, A., Kuehn, M. & Evans, M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature 323, 445–448 (1986).
Thomas, K. R. & Capecchi, M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51, 503–512 (1987).
Hakem, R. & Mak, T. W. Animal models of tumor-suppressor genes. Annu. Rev. Genet. 35, 209–241 (2001).
Christofori, G. & Hanahan, D. Molecular dissection of multi-stage tumorigenesis in transgenic mice. Semin. Cancer Biol. 5, 3–12 (1994).
Lewandoski, M. Conditional control of gene expression in the mouse. Nature Rev. Genet. 2, 743–755 (2001).
Sauer, B. Inducible gene targeting in mice using the Cre/lox system. Methods 14, 381–392 (1998).
Berns, A. Turning on tumors to study cancer progression. Nature Med. 5, 989–990 (1999).
Fisher, G. H. et al. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 18, 5253–5260 (1999).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Chang, C. & Werb, Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 11, S37–S43 (2001).
Olumi, A. F. et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 59, 5002–5011 (1999).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Coussens, L. M. et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382–1397 (1999).
Coussens, L. M., Tinkle, C. L., Hanahan, D., & Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103, 481–490 (2000).References 22 and 23 show that non-cell-autonomous factors, such as MMP-9 produced by inflammatory cells, actively participate in tumour formation. Interestingly, data in the latter study indicate that MMP-9 might promote the initial stages of tumour formation but inhibit later stages of tumour progression.
Hann, B. & Balmain, A. Building 'validated' mouse models of human cancer. Curr. Opin. Cell Biol. 13, 778–784 (2001).
Sherman, L., Wainwright, D., Ponta, H. & Herrlich, P. A splice variant of CD44 expressed in the apical ectodermal ridge presents fibroblast growth factors to limb mesenchyme and is required for limb outgrowth. Genes Dev. 12, 1058–1071 (1998).
Friedman, L. S. et al. Thymic lymphomas in mice with a truncating mutation in Brca2. Cancer Res. 58, 1338–1343 (1998).
Connor, F. et al. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nature Genet. 17, 423–430 (1997).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nature Genet. 22, 37–43 (1999).
Ludwig, T., Fisher, P., Murty, V. & Efstratiadis, A. Development of mammary adenocarcinomas by tissue-specific knockout of Brca2 in mice. Oncogene 20, 3937–3948 (2001).
Jonkers, J. et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nature Genet. 29, 418–425 (2001).References 28–30 show that conditional mutagenesis of Brca1 or Brca2 in mouse mammary-gland epithelium circumvents the embryonic lethality that is associated with germ-line mutations in these genes, and so allows modelling of hereditary breast cancer in mice. These conditional tumour models were subsequently used to establish a strong cooperation between Trp53 inactivation and loss of Brca1 (Ref. 28 ) or Brca2 (Ref. 30 ) in mammary tumorigenesis.
Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc. Natl Acad. Sci. USA 98, 1583–1588 (2001).
Marino, S., Vooijs, M., van Der, G. H., Jonkers, J. & Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000).
Meuwissen, R., Linn, S. C., van der Valk, M., Mooi, W. J. & Berns, A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene 20, 6551–6558 (2001).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001).Describes the generation of a mouse strain in which an activated Kras2 mutant is stochastically expressed in somatic cells as a result of a spontaneous recombination event. The resulting mice develop lung cancers that are metastatic in a p53-deficient background.
Federspiel, M. J., Bates, P., Young, J. A., Varmus, H. E. & Hughes, S. H. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc. Natl Acad. Sci. USA 91, 11241–11245 (1994).
Holland, E. C. Gliomagenesis: genetic alterations and mouse models. Nature Rev. Genet. 2, 120–129 (2001).
Dai, C. et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 15, 1913–1925 (2001).
Holland, E. C. et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature Genet. 25, 55–57 (2000).Describes the use of an in vivo transduction system using avian leukosis virus to introduce multiple mutations in neural cells and generate a mouse model for glioblastoma.
Holland, E. C., Hively, W. P., DePinho, R. A. & Varmus, H. E. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 12, 3675–3685 (1998).
Orsulic, S. et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1, 53–62 (2002).
Xu, K., Ma, H., McCown, T. J., Verma, I. M. & Kafri, T. Generation of a stable cell line producing high-titer self-inactivating lentiviral vectors. Mol. Ther. 3, 97–104 (2001).
Baron, U. & Bujard, H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 327, 401–421 (2000).
Eilers, M., Picard, D., Yamamoto, K. R. & Bishop, J. M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature 340, 66–68 (1989).
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Fisher, G. H. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001).References 45 and 46 validate activated Ras as a therapeutic target by showing that sustained expression of activated Kras2 is required for maintenance of lung tumours and melanomas, even in the presence of other oncogenic mutations.
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Blyth, K. et al. Sensitivity to myc-induced apoptosis is retained in spontaneous and transplanted lymphomas of CD2-mycER mice. Oncogene 19, 773–782 (2000).
Huettner, C. S., Zhang, P., Van Etten, R. A. & Tenen, D. G. Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nature Genet. 24, 57–60 (2000).
Pelengaris, S., Littlewood, T., Khan, M., Elia, G. & Evan, G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565–577 (1999).References 49 and 50 validate activated Myc as a therapeutic target by showing that sustained expression is required for maintenance of skin tumours and leukaemias.
Xie, W., Chow, L. T., Paterson, A. J., Chin, E. & Kudlow, J. E. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFα expression in transgenic mice. Oncogene 18, 3593–3607 (1999).
Tichelaar, J. W., Lu, W. & Whitsett, J. A. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275, 11858–11864 (2000).
Ewald, D. et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science 273, 1384–1386 (1996).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 21, 70–71 (1999).
Mao, X., Fujiwara, Y. & Orkin, S. H. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl Acad. Sci. USA 96, 5037–5042 (1999).
Mao, X., Fujiwara, Y., Chapdelaine, A., Yang, H. & Orkin, S. H. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 97, 324–326 (2001).
Awatramani, R., Soriano, P., Mai, J. J. & Dymecki, S. An Flp indicator mouse expressing alkaline phosphatase from the ROSA26 locus. Nature Genet. 29, 257–259 (2001).
Loonstra, A. et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl Acad. Sci. USA 98, 9209–9214 (2001).
Silver, D. P. & Livingston, D. M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell 8, 233–243 (2001).
Schmidt, E. E., Taylor, D. S., Prigge, J. R., Barnett, S. & Capecchi, M. R. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl Acad. Sci. USA 97, 13702–13707 (2000).
Pfeifer, A., Brandon, E. P., Kootstra, N., Gage, F. H. & Verma, I. M. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc. Natl Acad. Sci. USA 98, 11450–11455 (2001).References 58–61 document Cre-associated cytotoxicity in cultured cells and mice, and illustrate the need for carefully controlled experiments and the use of regulatable or self-inactivating Cre expression systems.
Thyagarajan, B., Guimaraes, M. J., Groth, A. C. & Calos, M. P. Mammalian genomes contain active recombinase recognition sites. Gene 244, 47–54 (2000).
Vooijs, M., Jonkers, J. & Berns, A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2, 292–297 (2001).
Jo, D. et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat. Biotechnol. 19, 929–933 (2001).
Shibata, H. et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278, 120–123 (1997).
Kononen, J. et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature Med. 4, 844–847 (1998).
Liyanage, M. et al. Multicolour spectral karyotyping of mouse chromosomes. Nature Genet. 14, 312–315 (1996).
Lindblad-Toh, K. et al. Large-scale discovery and genotyping of single-nucleotide polymorphisms in the mouse. Nature Genet. 24, 381–386 (2000).
Shi, Y. P. et al. DNA copy number changes associated with characteristic LOH in islet cell carcinomas of transgenic mice. Genes Chromosom. Cancer 19, 104–111 (1997).
Hodgson, G. et al. Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nature Genet. 29, 459–464 (2001).
Graveel, C. R., Jatkoe, T., Madore, S. J., Holt, A. L. & Farnham, P. J. Expression profiling and identification of novel genes in hepatocellular carcinomas. Oncogene 20, 2704–2712 (2001).
Kerlavage, A. et al. The Celera Discovery System. Nucleic Acids Res. 30, 129–136 (2002).
Benson, D. A. et al. GenBank. Nucleic Acids Res. 30, 17–20 (2002).
Hubbard, T. et al. The Ensembl genome database project. Nucleic Acids Res. 30, 38–41 (2002).
Liyanage, M. et al. Abnormal rearrangement within the α/δ T-cell receptor locus in lymphomas from Atm-deficient mice. Blood 96, 1940–1946 (2000).
Zimonjic, D. B., Pollock, J. L., Westervelt, P., Popescu, N. C. & Ley, T. J. Acquired, nonrandom chromosomal abnormalities associated with the development of acute promyelocytic leukemia in transgenic mice. Proc. Natl Acad. Sci. USA 97, 13306–13311 (2000).
Yu, Q., Geng, Y. & Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021 (2001).
Weissleder, R. Scaling down imaging: molecular mapping of cancer in mice. Nature Rev. Cancer 2, 11–18 (2002).
Vooijs, M., Jonkers, J., Lyons, S. & Berns, A. Non-invasive imaging of spontaneous Rb pathway-dependent tumors in mice. Cancer Res. 62, 1862–1867 (2002).
Gonzalez, F. J. & Kimura, S. Role of gene knockout mice in understanding the mechanisms of chemical toxicity and carcinogenesis. Cancer Lett. 143, 199–204 (1999).
Fischer, S. E., Wienholds, E. & Plasterk, R. H. Regulated transposition of a fish transposon in the mouse germ line. Proc. Natl Acad. Sci. USA 98, 6759–6764 (2001).
Ostertag, E. M., DeBerardinis, R. J., Kim, K.-S., Gerton, G. & Kazazian, H. H. Jr. Human L1 retrotransposition in germ cells of transgenic mice. Am. J. Hum. Genet. 67, A102 (2000).
Jonkers, J. & Berns, A. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim. Biophys. Acta 1287, 29–57 (1996).
Allen, J. D. & Berns, A. Complementation tagging of cooperating oncogenes in knockout mice. Semin. Cancer Biol. 7, 299–306 (1996).
Berns, A. et al. Identification and characterization of collaborating oncogenes in compound mutant mice. Cancer Res. 59, S1773–S1777 (1999). | PubMed |
Luo, G. et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 26, 424–429 (2000).
Balmain, A. & Harris, C. C. Carcinogenesis in mouse and human cells: parallels and paradoxes. Carcinogenesis 21, 371–377 (2000).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Rudolph, K. L., Millard, M., Bosenberg, M. W. & DePinho, R. A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nature Genet. 28, 155–159 (2001).
Giovannini, M. et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 14, 1617–1630 (2000).
Vooijs, M., van der Valk, M., te, R. H. & Berns, A. Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17, 1–12 (1998).
Holland, E. C. et al. Astrocytes give rise to oligodendro-gliomas and astrocytomas after gene transfer of polyoma virus middle T antigen in vivo. Am. J. Pathol. 157, 1031–1037 (2000).
Mak, T. W. et al. Brcal required for T cell lineage development but not TCR loci rearrangement. Nature Immunol. 1, 77–82 (2000).
Zhu, Y. et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15, 859–876 (2001).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Feil, R. et al. Ligand-activated site-specific recombination in mice. Proc. Natl Acad. Sci. USA 93, 10887–10890 (1996).
Akagi, K. et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 25, 1766–1773 (1997).
Lallemand-Breitenbach, V. et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 189, 1043–1052 (1999).
Rego, E. M., He, L. Z., Warrell, R. P. Jr, Wang, Z. G. & Pandolfi, P. P. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARα and PLZF-RARα oncoproteins. Proc. Natl Acad. Sci. USA 97, 10173–10178 (2000).
Bearss, D. J. et al. Genetic determinants of response to chemotherapy in transgenic mouse mammary and salivary tumors. Oncogene 19, 1114–1122 (2000).
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4A/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999).
Schmitt, C. A., Rosenthal, C. T. & Lowe, S. W. Genetic analysis of chemoresistance in primary murine lymphomas. Nature Med. 6, 1029–1035 (2000).
Menard, S. et al. Tamoxifen chemoprevention of a hormone-independent tumor in the proto-neu transgenic mice model. Cancer Res. 60, 273–275 (2000).
Jacoby, R. F. et al. Chemoprevention of spontaneous intestinal adenomas in the ApcMin mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 56, 710–714 (1996).
Torrance, C. J. et al. Combinatorial chemoprevention of intestinal neoplasia. Nature Med. 6, 1024–1028 (2000).
Gupta, S. et al. Chemoprevention of prostate carcinogenesis by α-difluoromethylornithine in TRAMP mice. Cancer Res. 60, 5125–5133 (2000).
Raghow, S., Kuliyev, E., Steakley, M., Greenberg, N. & Steiner, M. S. Efficacious chemoprevention of primary prostate cancer by flutamide in an autochthonous transgenic model. Cancer Res. 60, 4093–4097 (2000).
Arbeit, J. M. et al. Difluoromethylornithine chemoprevention of epidermal carcinogenesis in K14-HPV16 transgenic mice. Cancer Res. 59, 3610–3620 (1999).
Kohl, N. E. et al. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in Ras transgenic mice. Nature Med. 1, 792–797 (1995).
Barrington, R. E. et al. A farnesyltransferase inhibitor induces tumor regression in transgenic mice harboring multiple oncogenic mutations by mediating alterations in both cell cycle control and apoptosis. Mol. Cell Biol. 18, 85–92 (1998).
Mangues, R. et al. Antitumor effect of a farnesyl protein transferase inhibitor in mammary and lymphoid tumors overexpressing N-ras in transgenic mice. Cancer Res. 58, 1253–1259 (1998).
Norgaard, P. et al. Treatment with farnesyl-protein transferase inhibitor induces regression of mammary tumors in transforming growth factor (TGF)α and TGFα/neu transgenic mice by inhibition of mitogenic activity and induction of apoptosis. Clin. Cancer Res. 5, 35–42 (1999).
Mahgoub, N. et al. In vitro and in vivo effects of a farnesyltransferase inhibitor on Nf1-deficient hematopoietic cells. Blood 94, 2469–2476 (1999).
Omer, C. A. et al. Mouse mammary tumor virus-Ki-rasB transgenic mice develop mammary carcinomas that can be growth-inhibited by a farnesyl:protein transferase inhibitor. Cancer Res. 60, 2680–2688 (2000).
Trempus, C. S. et al. A farnesyl transferase inhibitor suppresses TPA-mediated skin tumor development without altering hyperplasia in the ras transgenic Tg. AC mouse. Mol. Carcinog. 27, 24–33 (2000).
Reichert, A., Heisterkamp, N., Daley, G. Q. & Groffen, J. Treatment of Bcr/Abl-positive acute lymphoblastic leukemia in P190 transgenic mice with the farnesyl transferase inhibitor SCH66336. Blood 97, 1399–1403 (2001).
Lenferink, A. E. et al. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-α bigenic mice. Proc. Natl Acad. Sci. USA 97, 9609–9614 (2000).
Podsypanina, K. et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc. Natl Acad. Sci. USA 98, 10320–10325 (2001).
Bergers, G. et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biol. 2, 737–744 (2000).
Bergers, G., Javaherian, K., Lo, K. M., Folkman, J. & Hanahan, D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284, 808–812 (1999).
Boehm, T., Folkman, J., Browder, T. & O'reilly, M. S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390, 404–407 (1997).
Acknowledgements
We apologize to all colleagues whose relevant contributions to the field could not be cited owing to space constraints. We thank P. Krimpenfort, A. Loonstra, R. Meuwissen and K. Quon for critical reading of the manuscript. Our studies reviewed in this article were supported by the Dutch Cancer Society and the Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
GenBank
LocusLink
Medscape DrugInfo
FURTHER READING
Cre transgenic and floxed gene database
Mouse Models for Human Cancer Consortium (MMHCC) homepage
Mouse Tumor Biology (MTB) database
Glossary
- PRISTANE
-
A carcinogenic saturated hydrocarbon (2,6,10,14-tetramethylpentadecane) that is purified from mineral oil.
- PROVIRUS
-
The duplex DNA form of the retroviral genome. The provirus is produced by reverse transcription of the RNA genome and is subsequently integrated into the chromosomal DNA of the infected host cell.
- HOMOLOGOUS RECOMBINATION
-
The process by which segments of DNA are exchanged between two DNA duplexes that share high sequence similarity.
- K14–HPV16
-
A transgenic mouse strain that expresses the human papillomavirus type 16 (HPV16) early-region genes, including the E6 and E7 oncogenes, under the control of the human keratin-14 promoter (K14), in basal keratinocytes. Invasive squamous carcinomas of the epidermis develop through characteristic stages.
- APICAL ECTODERMAL RIDGE
-
(AER). The layer of surface ectodermal cells that is at the apex of the embryonic limb bud. This layer exerts an inductive influence on the condensation of the underlying mesenchyme.
- MESENCHYME
-
Embryonic tissue that is composed of loosely organized, unpolarized cells of both mesodermal and ectodermal (for example, neural crest) origin, with a proteoglycan-rich extracellular matrix.
- HYPOMORPHIC ALLELE
-
An allele that results in a reduction, but not the elimination, of wild-type levels of gene product or activity, often causing a less severe phenotype than a loss-of-function (or null) allele.
- MEDULLOBLASTOMA
-
A brain tumour that consists of cells resembling the undifferentiated cells of the primitive medullary tube. Medulloblastomas are often located in the vermis of the cerebellum.
- EXTERNAL GRANULAR LAYER
-
The deepest of the three layers in the cerebellar cortex. It contains large numbers of granule cells, the dendrites of which form synapses with incoming mossy fibres in cerebellar glomeruli. The axons of the granule cells ascend into the molecular layer in which they bifurcate into fibres that form numerous synapses with the dendrites of Purkinje cells, basket cells and stellate cells.
- TRANSCRIPTIONAL TERMINATOR
-
A specific DNA sequence, represented at the end of a gene, that causes RNA polymerase to terminate transcription.
- ADENOVIRAL VECTOR
-
A gene-therapy vector that is derived from an adenovirus. Genes necessary for replication of the virus can be deleted to make replication-defective vectors.
- HERPES SIMPLEX VIRUS VP16
-
A potent transcriptional activator protein of herpes simplex virus. Various chimeric sequence-specific transcriptional activators have been engineered by fusing the VP16 transcriptional activation domain to the sequence-specific DNA-binding domains of other proteins.
- PAPILLOMATOSIS
-
Papillary projections of the epidermis that form a microscopically undulating surface.
- FORWARD SCREENS
-
Genetic screens that employ genome-wide saturation mutagenesis to obtain specific mutant phenotypes. The causal mutations can be subsequently identified by genetic and/or molecular approaches.
- ATAXIA-TELENGIECTASIA MUTATED
-
(ATM). Germ-line mutations in the ATM gene — involved in the DNA-damage response — are responsible for ataxia-telangiectasia/Louis–Bar syndrome, a familial recessive disease that is characterized by progressive cerebellar ataxia, oculocutaneous telangiectases and susceptibility to pulmonary infections.
- PML–RARα AND RARα–PML
-
Protein fusions between the promyelocytic leukaemia tumour suppressor (PML) and the retinoic-acid receptor-α (RARα). The fusion genes are associated with human acute promyelocytic leukaemias and are generated by reciprocal translocations of chromosomes 15 and 17.
- RIP-TAg
-
A transgenic mouse strain that expresses the simian virus 40 large T antigen (TAg) under the rat insulin II promoter (RIP) in the pancreatic islet cells. Carcinomas develop in the pancreatic islets and progress through characteristic stages.
- TELOMERE CRISIS
-
State of cells in which the progressive shortening of telomeres during successive cell divisions has impaired their capacity to protect the ends of chromosomal DNA, leading to end-to-end fusions and genomic instability.
Rights and permissions
About this article
Cite this article
Jonkers, J., Berns, A. Conditional mouse models of sporadic cancer. Nat Rev Cancer 2, 251–265 (2002). https://doi.org/10.1038/nrc777
Issue Date:
DOI: https://doi.org/10.1038/nrc777
This article is cited by
-
Meningioma animal models: a systematic review and meta-analysis
Journal of Translational Medicine (2023)
-
Ablative radiotherapy improves survival but does not cure autochthonous mouse models of prostate and colorectal cancer
Communications Medicine (2023)
-
T-ALL can evolve to oncogene independence
Leukemia (2021)
-
Loss of Smad4 promotes aggressive lung cancer metastasis by de-repression of PAK3 via miRNA regulation
Nature Communications (2021)
-
Preclinical mouse solid tumour models: status quo, challenges and perspectives
Nature Reviews Cancer (2017)