Key Points
-
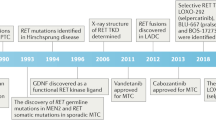
The RET receptor tyrosine kinase is required for the development of neural and genitourinary tissues, but deregulation of RET activity is an important contributor to several human cancers.
-
Activating point mutations in key functional motifs cause the inherited cancer syndrome multiple endocrine neoplasia type 2. RET rearrangements that lead to constitutively active cytosolic chimeric proteins occur somatically in sporadic carcinomas of the thyroid and the lung, and they have recently been found in patients with chronic myelomonocytic leukaemia, among others.
-
Expression and activation of wild-type RET is recognized in several tumour types, where it can contribute to tumour progression by multiple mechanisms. RET activity increases tumour regional invasion and perineural spread in carcinoma of the pancreas, and it is associated with the development of resistance to endocrine therapies in breast carcinoma.
-
RET activity contributes to tumour-associated inflammation by increasing levels of pro-inflammatory cytokines and chemokines in the tumour microenvironment, thereby recruiting primary immune cells and promoting tumour growth, invasive spread and/or distant metastasis.
-
Therapeutic approaches that target RET with small-molecule kinase inhibitors have proved to be clinically valuable in medullary thyroid cancer and are being evaluated for other cancers that are associated with RET mutations or increased RET expression.
-
Although anti-RET therapeutics are an important advance in managing RET-associated malignancies, RET also has important roles in the survival and maintenance of other tissues, such as neural cell types, which might have long-term implications for extended use of these therapies.
Abstract
The RET receptor tyrosine kinase is crucial for normal development but also contributes to pathologies that reflect both the loss and the gain of RET function. Activation of RET occurs via oncogenic mutations in familial and sporadic cancers — most notably, those of the thyroid and the lung. RET has also recently been implicated in the progression of breast and pancreatic tumours, among others, which makes it an attractive target for small-molecule kinase inhibitors as therapeutics. However, the complex roles of RET in homeostasis and survival of neural lineages and in tumour-associated inflammation might also suggest potential long-term pitfalls of broadly targeting RET.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ishizaka, Y. et al. Human ret proto-oncogene mapped to 10q11.2. Oncogene 4, 1519–1521 (1989).
Andrew, S. D. et al. Transcriptional repression of the RET proto-oncogene by a mitogen activated protein kinase-dependent signalling pathway. Gene 298, 9–19 (2002).
Leon, T. Y. et al. Transcriptional regulation of RET by Nkx2-1, Phox2b, Sox10, and Pax3. J. Pediatr. Surg. 44, 1904–1912 (2009).
Lang, D. & Epstein, J. A. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Hum. Mol. Genet. 12, 937–945 (2003).
Zhu, J., Garcia-Barcelo, M. M., Tam, P. K. & Lui, V. C. HOXB5 cooperates with NKX2-1 in the transcription of human RET. PLoS ONE 6, e20815 (2011).
Jeanpierre, C. et al. RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J. Med. Genet. 48, 497–504 (2011).
Diaz-Beya, M. et al. Acute myeloid leukemia with translocation (8;16)(p11;p13) and MYST3-CREBBP rearrangement harbors a distinctive microRNA signature targeting RET proto-oncogene. Leukemia 27, 595–603 (2013).
Emison, E. S. et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434, 857–863 (2005).
Yamaga, R. et al. RNA sequencing of MCF-7 breast cancer cells identifies novel estrogen-responsive genes with functional estrogen receptor-binding sites in the vicinity of their transcription start sites. Horm. Cancer 4, 222–232 (2013).
Wang, C., Mayer, J. A., Mazumdar, A. & Brown, P. H. The rearranged during transfection/papillary thyroid carcinoma tyrosine kinase is an estrogen-dependent gene required for the growth of estrogen receptor positive breast cancer cells. Breast Cancer Res. Treat. 133, 487–500 (2012).
Myers, S. M., Eng, C., Ponder, B. A. J. & Mulligan, L. M. Characterization of RET proto-oncogene 3′ splicing variants and polyadenylation sites: a novel C terminus for RET. Oncogene 11, 2039–2045 (1995).
Tahira, T., Ishizaka, Y., Itoh, F., Sugimura, T. & Nagao, M. Characterization of ret proto-oncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene 5, 97–102 (1990).
Carter, M. T. et al. Conservation of RET proto-oncogene splicing variants and implications for RET isoform function. Cytogenet. Cell Genet. 95, 169–176 (2001).
Takahashi, M. et al. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 3, 571–578 (1988).
Anders, J., Kjar, S. & Ibanez, C. F. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J. Biol. Chem. 276, 35808–35817 (2001).
Kjaer, S., Hanrahan, S., Totty, N. & McDonald, N. Q. Mammal-restricted elements predispose human RET to folding impairment by HSCR mutations. Nature Struct. Mol. Biol. 17, 726–731 (2010).
Amoresano, A. et al. Direct interactions among Ret, GDNF and GFRα1 molecules reveal new insights into the assembly of a functional three-protein complex. Cell. Signal. 17, 717–727 (2005).
Wang, X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim. Biophys. Acta 1834, 2205–2212 (2013).
Richardson, D. S. et al. Alternative splicing results in RET isoforms with distinct trafficking properties. Molec Biol. Cell 23, 3838–3850 (2012).
Runeberg-Roos, P., Virtanen, H. & Saarma, M. RET(MEN 2B) is active in the endoplasmic reticulum before reaching the cell surface. Oncogene 26, 7909–7915 (2007).
van Weering, D. H., Moen, T. C., Braakman, I., Baas, P. D. & Bos, J. L. Expression of the receptor tyrosine kinase Ret on the plasma membrane is dependent on calcium. J. Biol. Chem. 273, 12077–12081 (1998).
Hirata, Y., Shimokawa, N., Oh-hashi, K., Yu, Z. X. & Kiuchi, K. Acidification of the Golgi apparatus is indispensable for maturation but not for cell surface delivery of Ret. J. Neurochem. 115, 606–613 (2010).
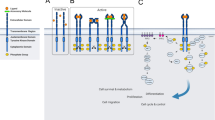
Arighi, E., Borrello, M. G. & Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 16, 441–467 (2005). This is an excellent in-depth review of RET-mediated downstream signalling.
Tansey, M. G., Baloh, R. H., Milbrandt, J. & Johnson, E. M. GFRα-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25, 611–623 (2000).
Airaksinen, M. S. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3, 383–394 (2002).
Airaksinen, M. S., Holm, L. & Hatinen, T. Evolution of the GDNF family ligands and receptors. Brain Behav. Evol. 68, 181–190 (2006).
Schalm, S. S., Ballif, B. A., Buchanan, S. M., Phillips, G. R. & Maniatis, T. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc. Natl. Acad. Sci. USA 107, 13894–13899 (2010).
Cockburn, J. G., Richardson, D. S., Gujral, T. S. & Mulligan, L. M. RET-mediated cell adhesion and migration require multiple integrin subunits. J. Clin. Endocrinol. Metab. 95, E342–E346 (2010).
Bonanomi, D. et al. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell 148, 568–582 (2012).
Tufro, A., Teichman, J., Banu, N. & Villegas, G. Crosstalk between VEGF-A/VEGFR2 and GDNF/RET signaling pathways. Biochem. Biophys. Res. Commun. 358, 410–416 (2007).
Esposito, C. L., D'Alessio, A., de Franciscis, V. & Cerchia, L. A cross-talk between TrkB and Ret tyrosine kinases receptors mediates neuroblastoma cells differentiation. PLoS ONE 3, e1643 (2008).
Popsueva, A. et al. GDNF promotes tubulogenesis of GFRα1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase. J. Cell Biol. 161, 119–129 (2003).
Ibanez, C. F. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 5, a009134 (2013).
Hayashi, H. et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19, 4469–4475 (2000).
Besset, V., Scott, R. P. & Ibanez, C. F. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J. Biol. Chem. 275, 39159–39166 (2000).
De Vita, G. & Melillo, R. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 60, 3727–3731 (2000).
Coulpier, M., Anders, J. & Ibanez, C. F. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J. Biol. Chem. 277, 1991–1999 (2002).
Segouffin-Cariou, C. & Billaud, M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-Kinase/AKT signaling pathway. J. Biol. Chem. 275, 3568–3576 (2000).
Jain, S., Encinas, M., Johnson, E. M. Jr. & Milbrandt, J. Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev. 20, 321–333 (2006).
Borrello, M. G. et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc. Natl Acad. Sci. USA 102, 14825–14830 (2005).
Jijiwa, M. et al. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells 13, 365–374 (2008).
Borrello, M. G. et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cγ. Mol. Cell. Biol. 16, 2151–2163 (1996).
Lundgren, T. K. et al. RET PLCγ phosphotyrosine binding domain regulates Ca2+ signaling and neocortical neuronal migration. PLoS ONE 7, e31258 (2012).
Encinas, M., Crowder, R. J., Milbrandt, J. & Johnson, E. M. Tyrosine 981, a novel Ret autophosphorylation site, binds c-Src to mediate neuronal survival. J. Biol. Chem. 279, 18262–18269 (2004).
Melillo, R. M. et al. Ret-mediated mitogenesis requires Src kinase activity. Cancer Res. 59, 1120–1126 (1999).
Schuringa, J. J. et al. MEN2A-RET-induced cellular transformation by activation of STAT3. Oncogene 20, 5350–5358 (2001).
Plaza-Menacho, I. et al. Ras/ERK1/2-mediated STAT3 Serine 727 phosphorylation by familiar medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity and transformation. J. Biol. Chem. 282, 6415–6424 (2007).
Fukuda, T., Kiuchi, K. & Takahashi, M. Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J. Biol. Chem. 277, 19114–19121 (2002).
Perrinjaquet, M., Vilar, M. & Ibanez, C. F. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J. Biol. Chem. 285, 31867–31875 (2010).
Asai, N. et al. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development 133, 4507–4516 (2006).
Plaza-Menacho, I. et al. Focal adhesion kinase (FAK) binds RET kinase via its FERM domain, priming a direct and reciprocal RET-FAK transactivation mechanism. J. Biol. Chem. 286, 17292–17302 (2011).
Gujral, T. S. et al. A novel RET kinase-β-catenin signaling pathway contributes to tumorigenesis in thyroid carcinoma. Cancer Res. 68, 1338–1346 (2008).
Castellone, M. D. et al. The β-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res. 69, 1867–1876 (2009).
Borrello, M. G. et al. Differential interaction of Enigma protein with the two RET isoforms. Biochem. Biophys. Res. Commun. 296, 515–522 (2002).
Schuetz, G. et al. The neuronal scaffold protein Shank3 mediates signaling and biological function of the receptor tyrosine kinase Ret in epithelial cells. J. Cell Biol. 167, 945–952 (2004).
Wong, A. et al. Phosphotyrosine 1062 is critical for the in vivo activity of the Ret9 receptor tyrosine kinase isoform. Mol. Cell. Biol. 25, 9661–9673 (2005).
Pachnis, V., Mankoo, B. & Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, 1005–1017 (1993).
Tsuzuki, T. et al. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene 10, 191–198 (1995).
Schuchardt, A., D'Agati, V., Pachnis, V. & Costantini, F. Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development 122, 1919–1929 (1996).
Chi, X. et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 (2009).
Davis, T. K., Hoshi, M. & Jain, S. To bud or not to bud: the RET perspective in CAKUT. Pediatr. Nephrol. http://dx.doi.org/10.1007/s00467-013-2606-5 (2013).
Meng, X. et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493 (2000).
Naughton, C. K., Jain, S., Strickland, A. M., Gupta, A. & Milbrandt, J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 74, 314–321 (2006).
Durbec, P. L., Larsson-Blomberg, L. B., Schuchardt, A., Costantini, F. & Pachnis, V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358 (1996).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994). This paper describes the first animal model to elucidate biological functions of RET.
Avantaggiato, V. et al. Developmental expression of the RET protooncogene. Cell Growth Differ. 5, 305–311 (1994).
de Graaff, E. et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 15, 2433–2444 (2001).
Pierchala, B. A., Milbrandt, J. & Johnson, E. M. Jr. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J. Neurosci. 26, 2777–2787 (2006).
Paratcha, G. & Ledda, F. GDNF and GFRα: a versatile molecular complex for developing neurons. Trends Neurosci. 31, 384–391 (2008).
Gattei, V. et al. Expression of the RET receptor tyrosine kinase and GDNFR-a in normal and leukemic human hematopoietic cells and stromal cells of the bone marrow microenvironment. Blood 89, 2925–2937 (1997).
Vargas-Leal, V. et al. Expression and function of glial cell line-derived neurotrophic factor family ligands and their receptors on human immune cells. J. Immunol. 175, 2301–2308 (2005).
Rusmini, M. et al. Induction of RET dependent and independent pro-inflammatory programs in human peripheral blood mononuclear cells from Hirschsprung patients. PLoS ONE 8, e59066 (2013).
Patel, A. et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal., 5, ra55 (2012).
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature 446, 547–551 (2007).
Amiel, J. et al. Hirschsprung disease, associated syndromes and genetics: a review. J. Med. Genet. 45, 1–14 (2008). This is a comprehensive review of loss-of-function RET mutations in HSCR.
Chatterjee, R. et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum. Genet. 131, 1725–1738 (2012).
Prato, A. P. et al. Hirschsprung disease and congenital anomalies of the kidney and urinary tract (CAKUT) a novel syndromic association. Medicine 88, 83–90 (2009).
Jain, S. The many faces of RET dysfunction in kidney. Organogenesis 5, 177–190 (2009).
Hwang, D. Y. et al. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. http://dx.doi.org/10.1038/ki.2013.508 (2014).
Donis-Keller, H. et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 2, 851–856 (1993).
Hofstra, R. M. W. et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367, 375–376 (1994).
Mulligan, L. M. et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363, 458–460 (1993).
Verga, U. et al. Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin. Endocrinol. 59, 156–161 (2003).
Kloos, R. T. et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19, 565–612 (2009).
Brandi, M. L. et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 86, 5658–5671 (2001).
Brauckhoff, M. et al. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann. Surg. http://dx.doi.org/10.1097/SLA.0b013e3182a6f43a (2013).
Choi, S. K., Yoon, S. R., Calabrese, P. & Arnheim, N. Positive selection for new disease mutations in the human germline: evidence from the heritable cancer syndrome multiple endocrine neoplasia type 2B. PLoS Genet. 8, e1002420 (2012).
Carlson, K. et al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am. J. Hum. Genet. 55, 1076–1082 (1994).
Margraf, R. L. et al. Multiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlations. Hum. Mutat. 30, 548–556 (2009). This paper describes a database of RET mutations and phenotypes, which is an important clinical and biological resource.
Wells, S. A. Jr., Pacini, F., Robinson, B. G. & Santoro, M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J. Clin. Endocrinol. Metab. 98, 3149–3164 (2013).
Mulligan, L. M. et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nature Genet. 6, 70–74 (1994).
Eng, C. et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2: International RET Mutation Consortium. JAMA. 276, 1575–1579 (1996). References 91–92 provide the first evidence of genotype–phenotype associations in MEN2, which have important implications for patient management.
Asai, N., Iwashita, T., Matsuyama, M. & Takahashi, M. Mecahanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol. Cell. Biol. 15, 1613–1619 (1995).
Quayle, F. J., Fialkowski, E. A., Benveniste, R. & Moley, J. F. Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery 142, 800–805 (2007).
Arighi, E. et al. Biological effects of the dual phenotypic Janus mutation of ret cosegregating with both multiple endocrine neoplasia type 2 and Hirschsprung's disease. Mol. Endocrinol. 18, 1004–1017 (2004).
Takahashi, M. et al. Co-segregation of MEN2 and Hirschsprung's disease: the same mutation of RET with both gain and loss-of-function? Hum. Mutat. 13, 331–336 (1999). This paper proposes mechanisms of loss-of-function and gain-of-function by a single RET mutation that provide insights into tissue-specific requirements for RET activity.
Lake, J. I. & Heuckeroth, R. O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G1–G24 (2013).
Frank-Raue, K. et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum. Mutat. 32, 51–58 (2011).
Mulligan, L. M. et al. Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum. Mol. Genet. 3, 2163–2167 (1994).
Gujral, T. S., Singh, V. K., Jia, Z. & Mulligan, L. M. Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2B. Cancer Res. 66, 10741–10749 (2006).
Knowles, P. P. et al. Structure and chemical inhibition of the RET tyrosine kinase domain. J. Biol. Chem. 281, 33577–33587 (2006).
Songyang, Z. et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 373, 536–539 (1995).
Jasim, S. et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid, 21, 189–192 (2011).
Eng, C. et al. Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res. 56, 2167–2170 (1996).
Machens, A. & Dralle, H. Familial prevalence and age of RET germline mutations: implications for screening. Clin. Endocrinol. 69, 81–87 (2008).
Bihan, H. et al. The clinical spectrum of RET proto-oncogene mutations in codon 790. Eur. J. Endocrinol. 169, 271–276 (2013).
Schulte, K. M. et al. The clinical spectrum of multiple endocrine neoplasia type 2a caused by the rare intracellular RET mutation S891A. J. Clin. Endocrinol. Metab. 95, E92–E97 (2010).
Signorini, P. S. et al. A ten-year clinical update of a large RET p. Gly533Cys kindred with medullary thyroid carcinoma emphasizes the need for an individualized assessment of affected relatives. Clin. Endocrinol. 80, 235–245 (2014).
Carlomagno, F. et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 23, 6056–6063 (2004).
Plaza Menacho, I. et al. RET-familial medullary thyroid carcinoma mutants Y791F and S891A activate a Src/JAK/STAT3 pathway, independent of glial cell line-derived neurotrophic factor. Cancer Res. 65, 1729–1737 (2005).
Iwashita, T. et al. Biological and biochemical properties of Ret with kinase domain mutations identified in multiple endocrine neoplasia type 2B and familial medullary thyroid carcinoma. Oncogene 18, 3919–3922 (1999).
Nakao, K. T. et al. Novel tandem germline RET proto-oncogene mutations in a patient with multiple endocrine neoplasia type 2B: report of a case and a literature review of tandem RET mutations with in silico analysis. Head Neck 35, E363–E368 (2013).
Cranston, A. N. et al. RET is constitutively activated by novel tandem mutations that alter the active site resulting in multiple endocrine neoplasia type 2B. Cancer Res. 66, 10179–10187 (2006).
Lantieri, F., Caroli, F., Ceccherini, I. & Griseri, P. The involvement of the RET variant G691S in medullary thyroid carcinoma enlightened by a meta-analysis study. Int. J. Cancer 132, 2808–2819 (2013).
Sawai, H. et al. The G691S RET polymorphism increases glial cell line-derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res. 65, 11536–11544 (2005).
Narita, N. et al. Functional RET G691S polymorphism in cutaneous malignant melanoma. Oncogene 28, 3058–3068 (2009).
Barr, J., Amato, C. M., Robinson, S. E., Kounalakis, N. & Robinson, W. A. The RET G691S polymorphism is a germline variant in desmoplastic malignant melanoma. Melanoma Res. 22, 92–95 (2012).
Robledo, M. et al. Polymorphisms G691S/S904S of RET as genetic modifiers of MEN 2A. Cancer Res. 63, 1814–1817 (2003).
Borrello, M. G. et al. Functional characterization of the MTC-associated germline RET-K666E mutation: evidence of oncogenic potential enhanced by the G691S polymorphism. Endocr. Relat. Cancer 18, 519–527 (2011).
Erlic, Z. et al. Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J. Clin. Endocrinol. Metab. 95, 308–313 (2010).
Berndt, I. et al. A new hot spot for mutations in the ret protooncogene causing familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2A. J. Clin. Endocrinol. Metab. 83, 770–774 (1998).
Seri, M. et al. Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum. Mutat. 9, 243–249 (1997).
Cosci, B. et al. In silico and in vitro analysis of rare germline allelic variants of RET oncogene associated with medullary thyroid cancer. Endocr. Relat. Cancer 18, 603–612 (2011).
Hyndman, B. D., Gujral, T. S., Krieger, J. R., Cockburn, J. G. & Mulligan, L. M. Multiple functional effects of RET kinase domain sequence variants in Hirschsprung disease. Hum. Mutat. 34, 132–142 (2013).
Mise, N., Drosten, M., Racek, T., Tannapfel, A. & Putzer, B. M. Evaluation of potential mechanisms underlying genotype-phenotype correlations in multiple endocrine neoplasia type 2. Oncogene 25, 6637–6647 (2006).
Toledo, R. A. et al. High penetrance of pheochromocytoma associated with the novel C634Y/Y791F double germline mutation in the RET protooncogene. J. Clin. Endocrinol. Metab. 95, 1318–1327 (2010).
Valente, F. O. et al. Comprehensive analysis of RET gene should be preformed in patients with MEN 2 syndrome and no apparent genotype-phenotype correlation: an appraisal of p. Y791F and p. C634Y RET mutations in five unrelated Brazilian families. J. Endocrinol. Invest. 36, 975–981 (2013).
Romei, C. & Elisei, R. RET/PTC translocations and clinico-pathological features in human papillary thyroid carcinoma. Front. Endocrinol. 3, 54 (2012).
Romei, C. et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J. Clin. Endocrinol. Metab. 97, E1758–E1765 (2012).
Leeman-Neill, R. J. et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer 119, 1792–1799 (2013).
Hamatani, K. et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 68, 7176–7182 (2008).
Bounacer, A. et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 15, 1263–1273 (1997).
Nikiforova, M. N. et al. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 290, 138–141 (2000). References 130–133 describe the contributions and mechanisms of environmental radiation exposure to RET rearrangements in PTC.
Dillon, L. W., Pierce, L. C., Lehman, C. E., Nikiforov, Y. E. & Wang, Y. H. DNA topoisomerases participate in fragility of the oncogene RET. PLoS ONE 8, e75741 (2013).
Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 13, 184–199 (2013).
Richardson, D. S., Gujral, T. S., Peng, S., Asa, S. L. & Mulligan, L. M. Transcript level modulates the inherent oncogenicity of RET/PTC oncoproteins. Cancer Res. 69, 4861–4869 (2009).
Kohno, T. et al. KIF5B-RET fusions in lung adenocarcinoma. Nature Med. 18, 375–377 (2012).
Li, F. et al. Identification of RET gene fusion by exon array analyses in “pan-negative” lung cancer from never smokers. Cell Res. 22, 928–931 (2012).
Lipson, D. et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nature Med. 18, 382–384 (2012).
Takeuchi, K. et al. RET, ROS1 and ALK fusions in lung cancer. Nature Med. 18, 378–381 (2012). References 137–140 highlight recent findings on chimeric RET proteins as oncogenes in carcinoma of the lung.
Drilon, A. et al. Response to Cabozantinib in Patients with RET Fusion-Positive Lung Adenocarcinomas. Cancer Discov. 3, 630–635 (2013).
Wang, R. et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 30, 4352–4359 (2012).
Nakayama, S. et al. Implication of expression of GDNF/Ret signalling components in differentiation of bone marrow haemopoietic cells. Br. J. Haematol. 105, 50–57 (1999).
Kohlmann, A. et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J. Clin. Oncol. 28, 3858–3865 (2010).
Ballerini, P. et al. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia 26, 2384–2389 (2012).
Ito, Y. et al. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery 138, 788–794 (2005).
Zeng, Q. et al. The relationship between overexpression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J. Int. Med. Res. 36, 656–664 (2008).
Gil, Z. et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J. Natl Cancer Inst. 102, 107–118 (2010).
Veit, C. et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 64, 5291–5300 (2004).
Cavel, O. et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 72, 5733–5743 (2012).
Plaza-Menacho, I. et al. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene 29, 4648–4657 (2010). This is an important demonstration of the contributions of RET expression to breast cancer tumorigenesis and endocrine resistance.
Esseghir, S. et al. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR α 1 receptor up-regulation in breast cancer. Cancer Res. 67, 11732–11741 (2007).
Morandi, A. et al. GDNF-RET signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res. 73, 3783–3795 (2013).
Gattelli, A. et al. Ret inhibition decreases growth and metastatic potential of estrogen receptor positive breast cancer cells. EMBO Mol. Med. 5, 1335–1350 (2013).
Kang, J. et al. Artemin is oncogenic for human mammary carcinoma cells. Oncogene 28, 2034–2045 (2009).
Boulay, A. et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERα pathway in breast cancer. Cancer Res. 68, 3743–3751 (2008).
Gattei, V. et al. Differential expression of the RET gene in human acute myeloid leukemia. Ann. Hematol. 77, 207–210 (1998).
Camos, M. et al. Gene expression profiling of acute myeloid leukemia with translocation t(8;16)(p11;p13) and MYST3-CREBBP rearrangement reveals a distinctive signature with a specific pattern of HOX gene expression. Cancer Res. 66, 6947–6954 (2006).
Mulligan, L. M. et al. Investigation of the genes for RET and its ligand complex GDNF/GFRa-1 in small cell lung carcinoma. Genes Chromosomes Cancer 21, 326–332 (1998).
Flavin, R. et al. RET protein expression in papillary renal cell carcinoma. Urol. Oncol. 30, 900–905 (2012).
Dawson, D. M. et al. Altered expression of RET proto-oncogene product in prostatic intraepithelial neoplasia and prostate cancer. J. Natl Cancer Inst. 90, 519–523 (1998).
Hofstra, R. M. W. et al. No mutations found by RET mutation scanning in sporadic and hereditary neuroblastoma. Hum. Genet. 97, 362–364 (1996).
Peterson, S. & Bogenmann, E. The RET and TRKA pathways collaborate to regulate neuroblastoma differentiation. Oncogene 23, 213–225 (2004).
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nature Rev. Cancer 3, 203–216 (2003).
Luo, Y. et al. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene 32, 2037–2047 (2013).
Sjoblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Diaz-Rodriguez, E. et al. Direct promoter induction of p19Arf by Pit-1 explains the dependence receptor RET/Pit-1/p53-induced apoptosis in the pituitary somatotroph cells. Oncogene 31, 2824–2835 (2012).
Garcia-Lavandeira, M. et al. Functional role of the RET dependence receptor, GFRa co-receptors and ligands in the pituitary. Front. Horm. Res. 38, 127–138 (2010).
Wells, S. A. Jr. et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012). This paper describes the outcomes of an important proof-of-principle clinical trial of vandetanib, the first tyrosine kinase inhibitor approved for treatment of advanced MTC.
Kurzrock, R. et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 29, 2660–2666 (2011).
Elisei, R. et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31, 3639–3646 (2013).
Borrello, M. G. et al. RET inhibition: implications in cancer therapy. Expert Opin. Ther. Targets 17, 403–419 (2013). This is a comprehensive review of multikinase inhibitors and their potential to target RET.
Sherman, S. I. Lessons learned and questions unanswered from use of multitargeted kinase inhibitors in medullary thyroid cancer. Oral Oncol. 49, 707–710 (2013).
Fox, E. et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin. Cancer Res. 19, 4239–4248 (2013).
De Falco, V. et al. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J. Clin. Endocrinol. Metab. 98, E811–E819 (2013).
Mologni, L., Redaelli, S., Morandi, A., Plaza-Menacho, I. & Gambacorti-Passerini, C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol. Cell Endocrinol. 377, 1–6 (2013).
Houvras, Y. Completing the Arc: targeted inhibition of RET in medullary thyroid cancer. J. Clin. Oncol. 30, 200–202 (2012).
Carhill, A. A. et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J. Clin. Endocrinol. Metab. 98, 31–42 (2013).
Barrenschee, M. et al. Site-specific gene expression and localization of growth factor ligand receptors RET, GFRα1 and GFRα2 in human adult colon. Cell Tissue Res. 354, 371–380 (2013).
Kramer, E. R. et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 5, e39 (2007).
Aron, L. et al. Pro-survival role for Parkinson's associated gene DJ-1 revealed in trophically impaired dopaminergic neurons. PLoS Biol. 8, e1000349 (2010).
Jain, S. et al. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J. Neurosci. 26, 11230–11238 (2006).
Golden, J. P. et al. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J. Neurosci. 30, 3983–3994 (2010).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Gild, M. L. et al. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr. Relat. Cancer 20, 659–667 (2013).
Dar, A. C., Das, T. K., Shokat, K. M. & Cagan, R. L. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature 486, 80–84 (2012). This paper describes novel phenotype-based strategies for identifying potential RET inhibitors as anticancer agents.
Grieco, M. et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60, 557–563 (1990).
Ossipov, M. H. Growth factors and neuropathic pain. Curr. Pain Headache Rep. 15, 185–192 (2011).
Carnicella, S. & Ron, D. GDNF—a potential target to treat addiction. Pharmacol. Ther. 122, 9–18 (2009).
Hoffer, B. J. & Harvey, B. K. Is GDNF beneficial in Parkinson disease? Nat. Rev. Neurol. 7, 600–602 (2011).
Ryu, H., Jeon, G. S., Cashman, N. R., Kowall, N. W. & Lee, J. Differential expression of c-Ret in motor neurons versus non-neuronal cells is linked to the pathogenesis of ALS. Lab Invest. 91, 342–352 (2011).
Krakora, D. et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol. Ther. 21, 1602–1610 (2013).
Tsui-Pierchala, B. A., Milbrandt, J. & Johnson, E. M. Jr. NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron 33, 261–273 (2002).
Skinner, M. A., Safford, S. D., Reeves, J. G., Jackson, M. E. & Freemerman, A. J. Renal aplasia in humans is associated with RET mutations. Am. J. Hum. Genet. 82, 344–351 (2008).
Brose, M. S. et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: The phase III DECISION trial. Journal Clinical Oncology 31, (Suppl.), a4 (2013).
Gild, M. L., Bullock, M., Robinson, B. G. & Clifton-Bligh, R. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat. Rev. Endocrinol. 7, 617–624 (2011).
Acknowledgements
The author thanks D. Richardson, M. Crupi and E. Lian for assistance in preparation of this manuscript. The author gratefully acknowledges financial support from The Cancer Research Society and the Carcinoid NeuroEndocrine Tumour Society of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- A marfanoid habitus
-
A physical phenotype that is reminiscent of individuals with Marfan syndrome. Features include elongated long bones and fingers, as well as hyperflexible joints.
- Pituitary somatotrophs
-
Secretory cells found in the anterior pituitary gland that produce growth hormone.
- Nociceptors
-
Sensory neurons that respond to noxious stimuli to initiate the sensation of pain.
- Amyotrophic lateral sclerosis
-
A progressive disorder of motor neuron degeneration that leads to muscle weakness and eventual atrophy.
Rights and permissions
About this article
Cite this article
Mulligan, L. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 14, 173–186 (2014). https://doi.org/10.1038/nrc3680
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3680
This article is cited by
-
Progress and challenges in RET-targeted cancer therapy
Frontiers of Medicine (2023)
-
Preclinical Pharmacokinetics and in vitro Metabolism of FHND5071, a Novel Selective RET Kinase Inhibitor
European Journal of Drug Metabolism and Pharmacokinetics (2023)
-
Looking for RET alterations in thyroid cancer: clinical relevance, methodology and timing
Endocrine (2023)
-
Clinical Characteristics and Responses to Immune Checkpoint Inhibitors in RET-Aberrant Digestive Tract Tumours
Targeted Oncology (2023)
-
Bimodal regulation of axonal transport by the GDNF-RET signalling axis in healthy and diseased motor neurons
Cell Death & Disease (2022)