Key Points
-
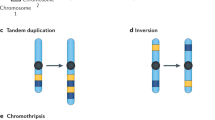
Chromosomal rearrangements that lead to oncogenic kinase activation are an emerging paradigm in epithelial cancers.
-
Ten different tyrosine kinases have currently been identified as fusion kinases in various different epithelial tumours. Tyrosine kinase gene rearrangements generally define molecularly distinct subsets of patients.
-
Cancers that harbour tyrosine kinase gene rearrangements express activated fusion kinases that drive the initiation and progression of malignancy. These cancers become dependent on (or 'addicted' to) continued signalling from the oncogenic fusion kinase.
-
Several tyrosine kinase inhibitors have been shown to be active in cancers that harbour specific tyrosine kinase fusions, which validates these fusion kinases as bona fide targets. In the case of anaplastic lymphoma kinase (ALK)-rearranged lung cancers, ALK inhibitors like crizotinib have become a standard therapy.
-
Multiplexed genetic analyses that include screening for tyrosine kinase fusions will help to accelerate the process of target discovery and clinical validation.
Abstract
Chromosomal rearrangements that lead to oncogenic kinase activation are observed in many epithelial cancers. These cancers express activated fusion kinases that drive the initiation and progression of malignancy, and often have a considerable response to small-molecule kinase inhibitors, which validates these fusion kinases as 'druggable' targets. In this Review, we examine the aetiologic, pathogenic and clinical features that are associated with cancers harbouring oncogenic fusion kinases, including anaplastic lymphoma kinase (ALK), ROS1 and RET. We discuss the clinical outcomes with targeted therapies and explore strategies to discover additional kinases that are activated by chromosomal rearrangements in solid tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Takahashi, M., Ritz, J. & Cooper, G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42, 581–588 (1985). This is the first report of RET rearrangement that leads to oncogenic kinase activation.
Tognon, C. et al. Expression of the ETV6–NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2, 367–376 (2002).
Soda, M. et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007). This is the first report of ALK gene rearrangements in a subset of patients with NSCLC.
Rikova, K. et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 (2007). These authors used a phosphoproteomics-based strategy to discover both ALK and ROS1 gene rearrangements in NSCLC.
Weinstein, I. B. Cancer. addiction to oncogenes — the Achilles heal of cancer. Science 297, 63–64 (2002).
Mitelman, F., Johansson, B. & Mertens, F. Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nature Genet. 36, 331–334 (2004).
Nikiforov, Y. E. RET/PTC rearrangement in thyroid tumors. Endocr. Pathol. 13, 3–16 (2002). This is a comprehensive review of RET rearrangements in papillary thyroid cancer.
Takeuchi, K. et al. RET, ROS1 and ALK fusions in lung cancer. Nature Med. 18, 378–381 (2012). Using a histopathology-based and molecular system, the authors screened tissue microarrays that contained more than1,500 lung cancers and identified multiple tyrosine kinase rearrangements, which included novel ROS1 and RET fusions.
Wang, R. et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 30, 4352–4359 (2012). This study shows that RET fusions define a distinct molecular subtype of NSCLC.
Bergethon, K. et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 30, 863–870 (2012). This study defines the clinicopathological features that are associated with ROS1 -rearranged NSCLC, and establishes ROS1as a target of crizotinib.
Kris, M. G. et al. Identification of driver mutations in tumor specimens from 1000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC). J. Clin. Oncol. Abstr. S29,CRA7506 (2011).
Bray, F., Ren, J. S., Masuyer, E. & Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 132, 1133–1145 (2013).
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 (2010).
Jabbour, E., Cortes, J. E., Ghanem, H., O'Brien, S. & Kantarjian, H. M. Targeted therapy in chronic myeloid leukemia. Expert Rev. Anticancer Ther. 8, 99–110 (2008).
Duffy, B. J. Jr & Fitzgerald, P. J. Cancer of the thyroid in children: a report of 28 cases. J. Clin. Endocrinol. Metab. 10, 1296–1308 (1950).
Kazakov, V. S., Demidchik, E. P. & Astakhova, L. N. Thyroid cancer after Chernobyl. Nature 359, 21–22 (1992).
Ito, T. et al. In vitro irradiation is able to cause RET oncogene rearrangement. Cancer Res. 53, 2940–2943 (1993).
Mizuno, T., Kyoizumi, S., Suzuki, T., Iwamoto, K. S. & Seyama, T. Continued expression of a tissue specific activated oncogene in the early steps of radiation-induced human thyroid carcinogenesis. Oncogene 15, 1455–1460 (1997).
Nikiforov, Y. E., Rowland, J. M., Bove, K. E., Monforte-Munoz, H. & Fagin, J. A. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 57, 1690–1694 (1997).
Fugazzola, L. et al. Oncogenic rearrangements of the RET proto-oncogene in papillary thyroid carcinomas from children exposed to the Chernobyl nuclear accident. Cancer Res. 55, 5617–5620 (1995).
Rabes, H. M. et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 6, 1093–1103 (2000).
Thomas, G. A. et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J. Clin. Endocrinol. Metab. 84, 4232–4238 (1999).
Ito, T. et al. Activated RET oncogene in thyroid cancers of children from areas contaminated by Chernobyl accident. Lancet 344, 259 (1994).
Klugbauer, S., Lengfelder, E., Demidchik, E. P. & Rabes, H. M. High prevalence of RET rearrangement in thyroid tumors of children from Belarus after the Chernobyl reactor accident. Oncogene 11, 2459–2467 (1995).
Ciampi, R. et al. Oncogenic AKAP9–BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115, 94–101 (2005).
Hamatani, K. et al. Rearranged anaplastic lymphoma kinase (ALK) gene in adult-onset papillary thyroid cancer amongst atomic bomb survivors. Thyroid 22, 1153–1159 (2012).
Ozasa, K. et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat. Res. 177, 229–243 (2012).
Gilbert, E. S. et al. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat. Res. 159, 161–173 (2003).
Swerdlow, A. J. et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. J. Clin. Oncol. 18, 498–509 (2000).
Rage, E. et al. Risk of lung cancer mortality in relation to lung doses among French uranium miners: follow-up 1956–1999. Radiat. Res. 177, 288–297 (2012).
Dano, L., Guilly, M. N., Dutrillaux, B. & Chevillard, S. Clonal evolution of a radon-induced rat lung tumor. Cancer Genet. Cytogenet. 125, 52–58 (2001).
Dano, L. et al. CGH analysis of radon-induced rat lung tumors indicates similarities with human lung cancers. Genes Chromosom. Cancer 29, 1–8 (2000).
Hubaux, R. et al. Arsenic, asbestos and radon: emerging players in lung tumorigenesis. Environ. Health 11, 89 (2012).
Mistry, A. R. et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N. Engl. J. Med. 352, 1529–1538 (2005).
Tsai, A. G. & Lieber, M. R. Mechanisms of chromosomal rearrangement in the human genome. BMC Genomics 11 (Suppl. 1), S1 (2010).
Shaw, A. T. et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4–ALK. J. Clin. Oncol. 27, 4247–4253 (2009).
Wong, D. W. et al. The EML4–ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115, 1723–1733 (2009).
Yoshida, A. et al. ROS1-rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am. J. Surg. Pathol. 37, 554–562 (2013).
Inamura, K. et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Modern Pathol. 22, 508–515 (2009).
Bongarzone, I. et al. RET/NTRK1 rearrangements in thyroid gland tumors of the papillary carcinoma family: correlation with clinicopathological features. Clin. Cancer Res. 4, 223–228 (1998).
Saad, A. G. et al. Proliferative activity of human thyroid cells in various age groups and its correlation with the risk of thyroid cancer after radiation exposure. J. Clin. Endocrinol. Metab. 91, 2672–2677 (2006).
Mani, R. S. & Chinnaiyan, A. M. Triggers for genomic rearrangements: insights into genomic, cellular and environmental influences. Nature Rev. Genet. 11, 819–829 (2010). This review summarizes the potential aetiological factors or triggers that contribute to chromosomal rearrangements.
Alt, F. W., Zhang, Y., Meng, F. L., Guo, C. & Schwer, B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell 152, 417–429 (2013).
Klein, I. A. et al. Translocation-capture sequencing reveals the extent and nature of chromosomal rearrangements in B lymphocytes. Cell 147, 95–106 (2011).
Chiarle, R. et al. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell 147, 107–119 (2011).
Barlow, J. H. et al. Identification of early replicating fragile sites that contribute to genome instability. Cell 152, 620–632 (2013).
Kim, N. & Jinks-Robertson, S. Transcription as a source of genome instability. Nature Rev. Genet. 13, 204–214 (2012).
Lin, C. et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell 139, 1069–1083 (2009).
Gandhi, M., Medvedovic, M., Stringer, J. R. & Nikiforov, Y. E. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene 25, 2360–2366 (2006).
Mani, R. S. et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science 326, 1230 (2009). This study shows that the juxtaposition of translocation partners can be mediated by transcription factors; specifically, in prostate cancer cells the androgen receptor induces proximity of the TMPRSS2 and ERG genomic loci, and in the presence of androgen and genotoxic stress it promotes DSBs and subsequent formation of the TMPRSS2–ERG fusion.
Bunting, S. F. & Nussenzweig, A. End-joining, translocations and cancer. Nature Rev. Cancer 13, 443–454 (2013). This review focuses on the factors that promote chromosomal rearrangements and, in particular, the role of non-homologous end-joining.
Nikiforova, M. N. et al. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 290, 138–141 (2000). This study shows that the spatial proximity of two chromosomal loci — RET and CCDC6 — facilitates the formation of a RET–CCDC6 fusion.
Hakim, O. et al. DNA damage defines sites of recurrent chromosomal translocations in B lymphocytes. Nature 484, 69–74 (2012).
Zhang, Y. et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148, 908–921 (2012).
Kalyana-Sundaram, S. et al. Gene fusions associated with recurrent amplicons represent a class of passenger aberrations in breast cancer. Neoplasia 14, 702–708 (2012).
Lipson, D. et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nature Med. 18, 382–384 (2012). Using a next-generation sequencing assay developed by Foundation Medicine, these authors identified chromosomal rearrangements involving ALK and RET in lung and colorectal cancers.
Kohno, T. et al. KIF5B-RET fusions in lung adenocarcinoma. Nature Med. 18, 375–377 (2012).
Charest, A. et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosom. Cancer 37, 58–71 (2003).
Chmielecki, J. et al. Targeted next-generation sequencing of DNA regions proximal to a conserved GXGXXG signaling motif enables systematic discovery of tyrosine kinase fusions in cancer. Nucleic Acids Res. 38, 6985–6996 (2010).
Chiarle, R., Voena, C., Ambrogio, C., Piva, R. & Inghirami, G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nature Rev. Cancer 8, 11–23 (2008).
Hernandez, L. et al. Diversity of genomic breakpoints in TFG-ALK translocations in anaplastic large cell lymphomas: identification of a new TFG-ALKXL chimeric gene with transforming activity. Am. J. Pathol. 160, 1487–1494 (2002).
Ou, S. H., Bartlett, C. H., Mino-Kenudson, M., Cui, J. & Iafrate, A. J. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist 17, 1351–1375 (2012).
To, K. F. et al. Detection of ALK rearrangement by immunohistochemistry in lung adenocarcinoma and the identification of a novel EML4-ALK variant. J. Thorac. Oncol. 8, 883–891 (2013).
Wu, Y. M. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013). These authors used 'integrative sequencing' (whole-exome sequencing, transcriptome sequencing and, as needed, low-pass genome sequencing) to discover a variety of FGFR fusions in diverse epithelial cancer types.
Knight, M. J., Leettola, C., Gingery, M., Li, H. & Bowie, J. U. A human sterile α-motif domain polymerizome. Protein Sci. 20, 1697–1706 (2011).
Peter, B. J. et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 (2004).
Mateja, A., Cierpicki, T., Paduch, M., Derewenda, Z. S. & Otlewski, J. The dimerization mechanism of LIS1 and its implication for proteins containing the LisH motif. J. Mol. Biol. 357, 621–631 (2006).
Seo, J. S. et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 22, 2109–2119 (2012).
Majewski, I. J. et al. Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centered RNA sequencing. J. Pathol. 230, 270–276 (2013).
Parker, B. C. et al. The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. J. Clin. Invest. 123, 855–865 (2013).
Monaco, C. et al. The RFG oligomerization domain mediates kinase activation and re-localization of the RET/PTC3 oncoprotein to the plasma membrane. Oncogene 20, 599–608 (2001).
Singh, D. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337, 1231–1235 (2012).
Sharma, S. V. & Settleman, J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 21, 3214–3231 (2007). This review examines the molecular mechanisms that underlie the phenomenon of oncogene addiction.
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature Rev. Cancer 9, 550–562 (2009).
Ley, R., Balmanno, K., Hadfield, K., Weston, C. & Cook, S. J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278, 18811–18816 (2003).
Costa, D. B. et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 4, 1669–1679; discussion 1680 (2007).
Cragg, M. S., Kuroda, J., Puthalakath, H., Huang, D. C. & Strasser, A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 4, 1681–1689; (2007).
Rahmani, M. et al. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood 114, 4507–4516 (2009).
Takezawa, K., Okamoto, I., Nishio, K., Janne, P. A. & Nakagawa, K. Role of ERK-BIM and STAT3-survivin signaling pathways in ALK inhibitor-induced apoptosis in EML4-ALK-positive lung cancer. Clin. Cancer Res. 17, 2140–2148 (2011).
Faber, A. C. et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 1, 352–365 (2011).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl. Med. 4, 120ra17 (2012).
Bean, G. R. et al. PUMA and BIM are required for oncogene inactivation-induced apoptosis. Sci. Signal. 6, ra20 (2013).
Faber, A. C. et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc. Natl Acad. Sci. USA 106, 19503–19508 (2009).
Ng, K. P. et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nature Med. 18, 521–528 (2012).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010). This landmark Phase I study established crizotinib as a highly effective and safe therapy for patients with advanced, ALK -rearranged NSCLC.
Zou, H. Y. et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 67, 4408–4417 (2007).
Christensen, J. G. et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 6, 3314–3322 (2007).
Cui, J. J. et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 54, 6342–6363 (2011).
McDermott, U. et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 68, 3389–3395 (2008).
Katayama, R. et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc. Natl Acad. Sci. USA 108, 7535–7540 (2011).
Camidge, D. R. et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 13, 1011–1019 (2012).
Kim, D. W. et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J. Clin. Oncol. 30 (Suppl.), 7533 (2012).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013). This is the first randomized study that compares crizotinib with chemotherapy in ALK -rearranged NSCLC.
Sakamoto, H. et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 19, 679–690 (2011).
Awad, M. M. et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N. Engl. J. Med. 368, 2395–2401 (2013).
Seto, T. et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 14, 590–598 (2013).
Ou, S. H. et al. Comparison of crizotinib (PF-02341066) pharmacokinetics between Asian and non-Asian patients with advanced malignancies. J. Thorac. Oncol. 5, S382 (2010).
Camidge, D. R. et al. First-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies: updated results. J. Clin. Oncol. Abstr. 31, 8031 (2013).
Shaw, A. T. et al. Clinical activity of the ALK inhibitor LDK378 in advanced, ALK-positive NSCLC. J. Clin. Oncol. Abstr. 31, 8010 (2013).
Doebele, R. C. et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin. Cancer Res. 18, 1472–1482 (2012).
Gainor, J. F. et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin. Cancer Res. 19, 4273–4281 (2013).
Shaw, A. T. et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. J. Clin. Oncol. Abstr. 30, 7508 (2012).
Acquaviva, J., Wong, R. & Charest, A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim. Biophys. Acta 1795, 37–52 (2009).
Ou, S. H. et al. Efficacy and safety of crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). J. Clin. Oncol. Abstr. 31, 8032 (2013).
Wells, S. A. et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012).
Schoffski, P. et al. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J. Clin. Oncol. 30 (Suppl.), 5508 (2012).
Sherman, S. I. et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N. Engl. J. Med. 359, 31–42 (2008).
Kloos, R. T. et al. Phase II trial of sorafenib in metastatic thyroid cancer. J. Clin. Oncol. 27, 1675–1684 (2009).
Drilon, A. et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 3, 630–635 (2013). This is the first paper to show clinical activity of a RET inhibitor in patients with RET -rearranged NSCLC, validating RET as a molecular target in lung cancer.
Gautschi, O. et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J. Thorac. Oncol. 8, e43–e44 (2013).
Xiao, S. et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t(8;13) leukaemia/lymphoma syndrome. Nature Genet. 18, 84–87 (1998).
Yagasaki, F. et al. Fusion of ETV6 to fibroblast growth factor receptor 3 in peripheral T-cell lymphoma with a t(4;12)(p16;p13) chromosomal translocation. Cancer Res. 61, 8371–8374 (2001).
Williams, S. V., Hurst, C. D. & Knowles, M. A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 22, 795–803 (2013).
Whitesell, L. & Lindquist, S. L. HSP90 and the chaperoning of cancer. Nature Rev. Cancer 5, 761–772 (2005). This is a comprehensive review of HSP90, and it includes a discussion of the potential therapeutic value of targeting HSP90 in cancer.
Bonvini, P., Gastaldi, T., Falini, B. & Rosolen, A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK+ CD30+ lymphoma cells by the Hsp90 antagonist 17-allylamino, 17-demethoxygeldanamycin. Cancer Res. 62, 1559–1566 (2002).
Sequist, L. V. et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J. Clin. Oncol. 28, 4953–4960 (2010).
Chen, Z. et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 70, 9827–9836 (2010).
Heuckmann, J. M. et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin. Cancer Res. 18, 4682–4690 (2012).
Sang, J. et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 3, 430–443 (2013).
Marsee, D. K. et al. Inhibition of heat shock protein 90, a novel RET/PTC1-associated protein, increases radioiodide accumulation in thyroid cells. J. Biol. Chem. 279, 43990–43997 (2004).
Socinski, M. A. et al. A multicenter Phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin. Cancer Res. 19, 3068–3077 (2013).
Felip, E. et al. Phase II activity of the hsp90 inhibitor AUY922 in patients with ALK-rearranged (ALK+) or EGFR-mutated advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 23 (Suppl. 9), 4380 (2012).
Palanisamy, N. et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nature Med. 16, 793–798 (2010).
Stephens, P. J. et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462, 1005–1010 (2009).
Huang, T., Karsy, M., Zhuge, J., Zhong, M. & Liu, D. B-Raf and the inhibitors: from bench to bedside. J. Hematol. Oncol. 6, 30 (2013).
Mino-Kenudson, M. et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin. Cancer Res. 16, 1561–1571 (2010).
Rimkunas, V. M. et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin. Cancer Res. 18, 4449–4457 (2012).
Gu, T. L. et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PloS ONE 6, e15640 (2011).
Lin, E. et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol. Cancer Res. 7, 1466–1476 (2009).
Li, F. et al. Identification of RET gene fusion by exon array analyses in “pan-negative” lung cancer from never smokers. Cell Res. 22, 928–931 (2012).
Fusco, A. et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 328, 170–172 (1987).
Giacomini, C. P. et al. Breakpoint analysis of transcriptional and genomic profiles uncovers novel gene fusions spanning multiple human cancer types. PLoS Genet. 9, e1003464 (2013). In this paper, by examining the transcript level or genomic DNA copy number transitions that occur within genes, the authors discover multiple gene fusions, which include a novel ROS1 fusion in angiosarcoma.
Ju, Y. S. et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 22, 436–445 (2012).
Doebele, R. C. et al. NTRK1 gene fusions as a novel oncogenic target in lung cancer. J. Clin. Oncol. Abstr. 31, 8023 (2013).
Chmielecki, J. et al. Systematic screen for tyrosine kinase rearrangements identifies a novel C6orf204-PDGFRB fusion in a patient with recurrent T-ALL and an associated myeloproliferative neoplasm. Genes Chromosomes Cancer 51, 54–65 (2012).
Takeuchi, K. et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin. Cancer Res. 15, 3143–3149 (2009).
Wong, D. W. et al. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer 117, 2709–2718 (2011).
Togashi, Y. et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PloS ONE 7, e31323 (2012).
Weickhardt, A. J. et al. ALK and ROS1 gene rearrangements detected in colorectal cancer (CRC) by fluorescence in situ hybridization (FISH). J. Clin. Oncol. Abstr. 31, 3545 (2013).
Jazii, F. R. et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer. World J. Gastroenterol. 12, 7104–7112 (2006).
Du, X. L. et al. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J. Mol. Med. 85, 863–875 (2007).
Debelenko, L. V. et al. Renal cell carcinoma with novel VCL–ALK fusion: new representative of ALK-associated tumor spectrum. Modern Pathol. 24, 430–442 (2011).
Sugawara, E. et al. Identification of anaplastic lymphoma kinase fusions in renal cancer: large-scale immunohistochemical screening by the intercalated antibody-enhanced polymer method. Cancer 118, 4427–4436 (2012).
Marino-Enriquez, A., Ou, W. B., Weldon, C. B., Fletcher, J. A. & Perez-Atayde, A. R. ALK rearrangement in sickle cell trait-associated renal medullary carcinoma. Genes Chromosomes Cancer 50, 146–153 (2011).
Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150, 1121–1134 (2012). The authors undertook whole-genome and transcriptome sequencing of 17 NSCLC tumours and identified numerous genetic alterations, including 14 gene fusions.
Suehara, Y. et al. Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clin. Cancer Res. 18, 6599–6608 (2012).
Birch, A. H. et al. Chromosome 3 anomalies investigated by genome wide SNP analysis of benign, low malignant potential and low grade ovarian serous tumours. PloS ONE 6, e28250 (2011).
Lee, J. et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer (2013).
Grieco, M. et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell 60, 557–563 (1990).
Bongarzone, I. et al. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RI alpha of cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 13, 358–366 (1993).
Bongarzone, I. et al. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 54, 2979–2985 (1994).
Klugbauer, S., Demidchik, E. P., Lengfelder, E. & Rabes, H. M. Detection of a novel type of RET rearrangement (PTC5) in thyroid carcinomas after Chernobyl and analysis of the involved RET-fused gene RFG5. Cancer Res. 58, 198–203 (1998).
Nakata, T. et al. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11;p13) in a papillary thyroid carcinoma. Genes Chromosomes Cancer 25, 97–103 (1999).
Corvi, R., Berger, N., Balczon, R. & Romeo, G. RET/PCM-1: a novel fusion gene in papillary thyroid carcinoma. Oncogene 19, 4236–4242 (2000).
Klugbauer, S. & Rabes, H. M. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene 18, 4388–4393 (1999).
Salassidis, K. et al. Translocation t(10;14)(q11.2:q22.1) fusing the kinetin to the RET gene creates a novel rearranged form (PTC8) of the RET proto-oncogene in radiation-induced childhood papillary thyroid carcinoma. Cancer Res. 60, 2786–2789 (2000).
Saenko, V. et al. Novel tumorigenic rearrangement, Delta rfp/ret, in a papillary thyroid carcinoma from externally irradiated patient. Mut. Res. 527, 81–90 (2003).
Ciampi, R., Giordano, T. J., Wikenheiser-Brokamp, K., Koenig, R. J. & Nikiforov, Y. E. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocr.-Related Cancer 14, 445–452 (2007).
Martin-Zanca, D., Hughes, S. H. & Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319, 743–748 (1986).
Butti, M. G. et al. A sequence analysis of the genomic regions involved in the rearrangements between TPM3 and NTRK1 genes producing TRK oncogenes in papillary thyroid carcinomas. Genomics 28, 15–24 (1995).
Greco, A. et al. TRK-T1 is a novel oncogene formed by the fusion of TPR and TRK genes in human papillary thyroid carcinomas. Oncogene 7, 237–242 (1992).
Greco, A. et al. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled-coil domain. Mol. Cell. Biol. 15, 6118–6127 (1995).
Skalova, A. et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 34, 599–608 (2010).
Marsilje, T. H. et al. Synthesis, structure-activity relationships and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in Phase 1 and 2 clinical trials. J. Med. Chem. 56, 5675–5690 (2013).
Patnaik, A. et al. Pharmacokinetics and safety of an oral ALK inhibitor, ASP3026, observed in a phase I dose escalation trial. J. Clin. Oncol. Abstr. 31, 2602 (2013).
Lovly, C. M. et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res. 71, 4920–4931 (2011).
Wilcoxen, K. M. et al. Characterization of a novel series of potent, selective inhibitors of wild type and mutant/fusion anaplastic lymphoma kinase. Cancer Res. 72 (Suppl. 1), 1795 (2012).
Gozgit, J. M. et al. Ponatinib is a highly potent inhibitor of activated variants of RET found in MTC and NSCLC. Cancer Res. 73 (Suppl. 1), 2084 (2013).
Plaza-Menacho, I. et al. Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J. Biol. Chem. 282, 29230–29240 (2007).
Smyth, E. C. et al. FGFR: proof-of-concept study of AZD4547 in patients with FGFR1 or FGFR2 amplified tumors. J. Clin. Oncol. Abstr. 31, TPS2626 (2013).
Gozgit, J. M. et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol. Cancer Ther. 11, 690–699 (2012).
Chase, A., Grand, F. H. & Cross, N. C. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood 110, 3729–3734 (2007).
Guagnano, V. et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov. 2, 1118–1133 (2012).
Bello, E. et al. E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 71, 1396–1405 (2011).
Yu, Y. et al. Exploratory biomarker discovery for clinical development of ARQ 087, a potent pan-FGFR kinase inhibitor. Cancer Res. 71 (Suppl. 1), 3571 (2011).
Acknowledgements
The authors are supported by grants from the US National Institutes of Health (NIH) 5R01CA164273-02 (A.T.S. and J.A.E.), V Foundation for Cancer Research (A.T.S. and J.A.E.), and Uniting Against Lung Cancer (A.T.S.). A.T.S. is the Charles W. and Jennifer C. Johnson Koch Institute Clinical Investigator. The authors thank I. Klein for his helpful comments, and Be a Piece of the Solution and the Evan Spirito Memorial Foundation for support of lung cancer research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
A.T.S. is a consultant for Pfizer, Novartis, Ariad, Chugai, Daiichi-sankyo, Genentech. J.A.E is a consultant for Agios, AstraZeneca, Cell Signaling Technology, Chugai, GlaxoSmithKline, Novartis and Sanofi Aventis; research funding from Novartis, Sanofi Aventis, AstraZeneca, GlaxoSmithKline; equity in Agios and Gatekeeper.
Related links
Supplementary information
Supplementary information S1 (table)
Hsp90-Based Therapeutic Strategies for Kinase Fusion-Driven Cancers (PDF 219 kb)
Rights and permissions
About this article
Cite this article
Shaw, A., Hsu, P., Awad, M. et al. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer 13, 772–787 (2013). https://doi.org/10.1038/nrc3612
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3612
This article is cited by
-
The role of CRAF in cancer progression: from molecular mechanisms to precision therapies
Nature Reviews Cancer (2024)
-
Establishment of an acquired lorlatinib-resistant cell line of non-small cell lung cancer and its mediated resistance mechanism
Clinical and Translational Oncology (2022)
-
Efficacy and safety exposure–response analyses of entrectinib in patients with advanced or metastatic solid tumors
Cancer Chemotherapy and Pharmacology (2022)
-
Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements in the NCI-MATCH trial
npj Precision Oncology (2022)
-
Genomic and biological study of fusion genes as resistance mechanisms to EGFR inhibitors
Nature Communications (2022)