Key Points
-
Macrophage-stimulating protein (MSP; also known as MST1 and hepatocye growth factor-like (HGFL)), is a serum-derived growth factor that activates the RON receptor tyrosine kinase. RON signalling is essential for the survival of embryos during the peri-implantation period. Cancer cells use MSP–RON signalling for survival, migration, angiogenesis and chemoresistance, which has pathogenic implications.
-
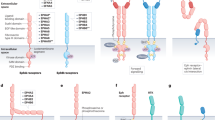
Crystal structure analyses have shown that a central cleft harbouring three amino acid residues in the putative catalytic site of the MSP β-chain interacts with an interface created by two RON semaphorin (SEMA) domains. This supports a model in which one MSP molecule binds two RON molecules to cause receptor dimerization and activation.
-
Aberrant RON activation, which is induced by overexpression of protein and the generation of oncogenic isoforms and is indicated by the persistent activation of multi-intracellular signalling cascades, occurs in various types of cancers. RON signalling is also necessary for cancer cell growth and survival. These features render RON as a drug target for cancer therapy.
-
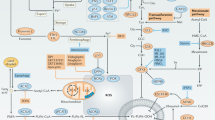
MSP–RON signalling activates two classical signalling pathways, RAS–ERK and PI3K–AKT, both of which interact with various other pathways to create a complex signalling network. RON also crosstalks with other receptor tyrosine kinases and viral oncoproteins. Such intricate interactions highlight the importance of RON signalling in cancer pathogenesis.
-
Overexpression of RON in primary tumours such as colon and breast cancer is predictive of patient survival and correlates with clinical and pathological parameters.
-
Studies from mouse xenograft models have revealed that inhibition of RON by tyrosine kinase inhibitors and monoclonal antibodies blocks tumour growth. However, combinations with chemoagents achieve maximum therapeutic benefit. The tyrosine kinase inhibitor BMS-777607 and the RON-specific antibody narnatumab are currently in Phase I clinical trials.
Abstract
Since the discovery of MSP (macrophage-stimulating protein; also known as MST1 and hepatocyte growth factor-like (HGFL)) as the ligand for the receptor tyrosine kinase RON (also known as MST1R) in the early 1990s, the roles of this signalling axis in cancer pathogenesis has been extensively studied in various model systems. Both in vitro and in vivo evidence has revealed that MSP–RON signalling is important for the invasive growth of different types of cancers. Currently, small-molecule inhibitors and antibodies blocking RON signalling are under investigation. Substantial responses have been achieved in human tumour xenograft models, laying the foundation for clinical validation. In this Review, we discuss recent advances that demonstrate the importance of MSP–RON signalling in cancer and its potential as a therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Leonard, E. J. & Skeel, A. H. Isolation of macrophage stimulating protein (MSP) from human serum. Exp. Cell. Res. 114, 117–126 (1978).
Leonard, E. J. & Skeel, A. H. Enhancement of spreading, phagocytosis and chemotaxis by macrophage stimulating protein (MSP). Adv. Exp. Med. Biol. 121B, 181–194 (1979).
Skeel, A. et al. Macrophage stimulating protein: purification, partial amino acid sequence, and cellular activity. J. Exp. Med. 173, 1227–1234 (1991). References 1 and 3 describe a serum protein that stimulates spreading, migration and phagocytosis of murine peritoneal resident macrophages and its subsequent purification to homology from human plasma.
Han, S. Stuart, L. A., & Degen, S. J. Characterization of the DNF15S2 locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry 30, 9768–9780 (1991).
Degen, S. J., Stuart, L. A., Han, S. & Jamison, C. S. Characterization of the mouse cDNA and gene coding for a hepatocyte growth factor-like protein: expression during development. Biochemistry 30, 9781–9791 (1991).
Yoshimura, T., Yuhki, N., Wang, M. H., Skeel, A. & Leonard, E. J. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J. Biol. Chem. 268, 15461–15468 (1993). References 4 and 6 report the molecular cloning of human MSP cDNA, its gene structure and chromosome location. The deduced sequences indicate that MSP belongs to the HGF/SF family.
Ronsin, C., Muscatelli, F., Mattei, M. G. & Breathnach, R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene 8, 1195–1202 (1993). This article reports a novel receptor tyrosine kinase (RON) with structural similarity to the MET proto-oncogene. The finding establishes RON as a member of the MET subfamily of human RTKs.
Gherardi, E., Sharpe, M., Lane, K., Sirulnik, A. & Stoker, M. Hepatocyte growth factor/scatter factor (HGF/SF), the c-met receptor and the behaviour of epithelial cells. Symp. Soc. Exp. Biol. 47, 163–181 (1993).
Park, M. et al. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl Acad. Sci. USA 84, 6379–6383 (1987).
Bottaro, D. P. et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251, 802–804 (1991).
Iwama, A., Okano, K., Sudo, T., Matsudam, Y. & Suda, T. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood 83, 3160–3169 (1994).
De Maria, R. et al. Feline STK gene expression in mammary carcinomas. Oncogene 21, 1785–1790 (2002).
Bassett, D. I. Identification and developmental expression of a macrophage stimulating 1/ hepatocyte growth factor-like 1 orthologous in the zebrafish. Dev. Genes Evol. 213, 360–362 (2003).
Huitema, L. F. et al. Macrophage-stimulating protein and calcium homeostasis in zebrafish. FASEB J. 26, 4092–4101 (2012).
Théry, C., Sharpe, M. J., Batley, S. J., Stern, C. D. & Gherardi, E. Expression of HGF/SF, HGF1/MSP, and c-met suggests new functions during early chick development. Dev. Genet. 17, 90–101 (1995).
Huff, J. L., Jelinek, M. A., Jamieson, T. A. & Parsons, J. T. Expression and maturation of the cellular sea receptor, a member of the hepatocyte growth factor (HGF) receptor family of protein tyrosine kinases. Oncogene 12, 299–307 (1996).
Wahl, R. C. et al. Chicken macrophage stimulating protein is a ligand of the receptor protein-tyrosine kinase Sea. J. Biol. Chem. 274, 26361–26368 (1999).
Nakamura, T. et al. Cloning and expression of Xenopus HGF-like protein (HLP) and Ron/HLP receptor implicate their involvement in early neural development. Biochem. Biophys. Res. Commun. 224, 564–573 (1996).
Hayman, M. J., Kitchener, G., Vogt, P. K. & Beug, H. The putative transforming protein of S13 avian erythroblastosis virus is a transmembrane glycoprotein with an associated protein kinase activity. Proc. Natl Acad. Sci. USA 82, 8237–8241 (1985).
T. K.Ith, D. R., Vogt, P. K. & Hayman, M. J. The v-sea oncogene of avian erythroblastosis retrovirus S13: another member of the protein-tyrosine kinase gene family. Proc. Natl Acad. Sci. USA 86, 5291–5295 (1989).
Wang, M. H. et al. Identification of the Ron gene product as the receptor for the human macrophage stimulating protein. Science 266, 117–119 (1994).
Gaudino, G. et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 13, 3524–3532 (1994). References 21 and 22 describe a series of biochemical and biological experiments that demonstrate human MSP as the specific ligand for RON. The finding establishes the MSP–RON signalling axis.
Wang, M. H., Iwama, A., Skeel, A., Suda, T. & Leonard, E. J. The murine stk gene product, a transmembrane protein tyrosine kinase, is a receptor for macrophage-stimulating protein. Proc. Natl Acad. Sci. USA 92, 3933–3937 (1995).
Bezerra, J. A., Carrick, T. L., Degen, J. L., Witte, D. & Degen, S. J. Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J. Clin. Invest. 101, 1175–1183 (1998).
Gaudino, G. et al. The proto-oncogene RON is involved in development of epithelial, bone and neuro-endocrine tissues. Oncogene 11, 2627–2637 (1995).
Quantin, B., Schuhbaur, B., Gesnel, M. C., Doll'e, P. & Breathnach, R. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev. Dyn. 204, 383–390 (1995).
Muraoka, R. S. et al. The Ron/STK receptor tyrosine kinase is essential for peri-implantation development in the mouse. J. Clin. Invest. 103, 1277–1285 (1999). This was the first report showing that complete inactivation of RON results in embryonic death in the early stage of mouse development. However, hemizygous mice (RON+/−) are healthy and grow to adulthood.
Krawczyk, M. et al. Macrophage stimulating protein variation enhances the risk of sporadic extrahepatic cholangiocarcinoma. Dig. Liver Dis. 16 Feb 2013 (doi:10.1016/j.dld.2012.12.017).
Häuser, F. et al. Macrophage-stimulating protein polymorphism rs3197999 is associated with a gain of function: implications for inflammatory bowel disease. Genes Immun. 13, 321–327 (2012).
Wang, M. H., Lee, W., Luo, Y. L., Weis, M. T. & Yao, H. P. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumorigenic phenotypes in thyroid cancer cells. J. Pathol. 213, 402–411 (2007).
Thomas, R. M. et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 67, 6075–6082 (2007).
Kanteti, R. et al. Differential expression of RON in small and non-small cell lung cancers. Genes Chromosomes Cancer 51, 841–851 (2012).
Camp, E. R. et al. Tyrosine kinase receptor RON in human pancreatic cancer: expression, function, and validation as a target. Cancer 109, 1030–1039 (2007).
Maggiora, P. et al. Overexpression of the RON gene in human breast carcinoma. Oncogene 16, 2927–2933 (1998).
Catenacci, D. V. et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol. Ther. 12, 9–46 (2011). This was the first report showing high frequencies of RON amplification and point mutations in primary gastro-oesophageal adenocarcinoma samples and established cell lines.
Ren, X. et al. Expression and mutational status of RON in neoplastic lesions of the breast: analysis of MSP/RON signaling in ductal carcinoma in situ and invasive ductal carcinoma. APMIS 120, 358–367 (2012).
Chou, Y. C. et al. Involvement of recepteur d'origine nantais receptor tyrosine kinase in Epstein-Barr virus-associated nasopharyngeal carcinoma and its metastasis. Am. J. Pathol. 181, 1773–1781 (2012).
Collesi, C., Santoro, M. M., Gaudino, G. & Comoglio, P. M. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol. Cell. Biol. 16, 5518–5526 (1996).
Santoro, M. M., Collesi, C., Grisendi, S., Gaudino, G. & Comoglio, P. M. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol. Cell. Biol. 16, 7072–7083 (1996). References 38 and 39 describe the discovery of the first splicing isoform of RON (RONΔ165) and the finding that in vitro wild-type RON is not a cell-transforming agent in a classical mouse fibroblast-transforming assay.
Zhou, Y. Q., He, C., Chen, Y. Q., Wang, D. & Wang, M. H. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene 22, 186–197 (2003).
Liu, X. et al. Short-form ron promotes spontaneous breast cancer metastasis through interaction with phosphoinositide 3-kinase. Genes Cancer 2, 753–762 (2011).
Eckerich, C. et al. RON receptor tyrosine kinase in human gliomas: expression, function, and identification of a novel soluble splice variant. J. Neurochem. 109, 969–980 (2009).
Ma, Q. et al. Deletion or insertion in the first immunoglobulin-plexin-transcription (IPT) domain differentially regulates expression and tumorigenic activities of RON receptor Tyrosine Kinase. Mol. Cancer 9, 307–316 (2010).
Wei, X. et al. Altered exon usage in the juxtamembrane domain of mouse and human RON regulates receptor activity and signaling specificity. J. Biol. Chem. 280, 40241–44051 (2005).
Ghigna, C. et al. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron proto-oncogene. Mol. Cell. 20, 881–890 (2005).
Fialin, C. et al. The short form of RON is expressed in acute myeloid leukemia and sensitizes leukemic cells to cMET inhibitors. Leukemia 2013 27, 325–335 (2013).
Bardella, C. et al. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 64, 5154–5161 (2004).
Wang, M. H., Kurtz, A. L. & Chen, Y. Q. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis 21, 1507–1512 (2000).
Wang, J. et al. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J. Biol. Chem. 284, 10912–10922 (2009).
Wang, D., Shen, Q., Chen, Y. Q. & Wang, M. H. Collaborative activities of macrophage-stimulating protein and transforming growth factor-β1 in induction of epithelial to mesenchymal transition: roles of the RON receptor tyrosine kinase. Oncogene 23, 1668–1680 (2004). This paper reports RON signalling in coordination with TGFβ1 to induce complete EMT in MDCK cells.
Chen, Y. Q., Zhou, Y. Q., Fisher, J. H. & Wang, M. H. Targeted expression of the receptor tyrosine kinase RON in distal lung epithelial cells results in multiple tumor formation: oncogenic potential of RON in vivo. Oncogene 21, 6382–6386 (2002). This was the first report showing that targeted RON expression in distal lung epithelial cells in a mouse transgenic model causes multiple lung adenomas and their subsequent progression towards lung adenocarcinomas.
Sato, S. et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin. Exp. Metastasis 30, 333–344 (2012).
Thobe, M. N. et al. The Ron receptor promotes prostate tumor growth in the TRAMP mouse model. Oncogene 30, 4990–4998 (2011).
Thobe, M. N., Gurusamy, D., Pathrose, P. & Waltz, S. E. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene. 29, 214–226 (2010). References 53 and 54 describe a direct correlation between RON overexpression and increased production of angiogenic VEGF and the chemokine CXCL2 in prostate cancer cells, suggesting RON signalling as a mechanism for tumour angiogenesis.
McClaine, R. J., Marshall, A. M., Wagh, P. K. & Waltz, S. E. Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia 12, 650–658 (2010).
Logan-Collins, J. et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 70, 1130–1140 (2010). References 55 and 56 describe activation of RON signalling as an important mechanism that confers chemoresistance in both pancreatic and breast cancer cells.
Chan, E. L., Peace, B. E., Collins, M. H., Toney-Earley, K. & Waltz, S. E. Ron tyrosine kinase receptor regulates papilloma growth and malignant conversion in a murine model of skin carcinogenesis. Oncogene 24, 479–488 (2005).
Zinser, G. M. et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with β-catenin activation. Cancer Res. 66, 11967–11974 (2006).
Welm, A. L. et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc. Natl Acad. Sci. USA 104, 7570–7575 (2007).
Feres, K. J., Ischenko, I. & Hayman, M. J. The RON receptor tyrosine kinase promotes MSP-independent cell spreading and survival in breast epithelial cells. Oncogene 28, 279–288 (2009).
Wang, M. H., Yoshimura, T., Skeel, A. & Leonard, E. J. Proteolytic conversion of single chain precursor macrophage-stimulating protein to a biologically active heterodimer by contact enzymes of the coagulation cascade. J. Biol. Chem. 269, 3436–3440 (1994).
Wang, M. H. et al. Proteolytic activation of single-chain precursor macrophage-stimulating protein by nerve growth factor-γ and epidermal growth factor-binding protein, members of the kallikrein family. J. Biol. Chem. 269, 13806–13810 (1994).
Kawaguchi, M., Orikawa, H., Baba, T., Fukushima, T. & Kataoka, H. Hepatocyte growth factor activator is a serum activator of single-chain precursor macrophage-stimulating protein. FEBS J. 276, 3481–3490 (2009).
Ganesan, R. et al. Proteolytic activation of pro-macrophage-stimulating protein by hepsin. Mol. Cancer Res. 9, 1175–1186 (2011).
Wang, M. H. et al. Macrophage stimulating protein (MSP) binds to its receptor via the MSP β chain. J. Biol. Chem. 272, 16999–17004 (1997).
Danilkovitch, A., Miller, M. & Leonard, E. J. Interaction of macrophage-stimulating protein with its receptor. Residues critical for β chain binding and evidence for independent α chain binding. J. Biol. Chem. 274, 29937–29943 (1999). References 65 and 66 describe discoveries that the high affinity-binding site for RON is located in the MSP β-chain and the MSP α-chain harbours a low-affinity binding site. The binding of both MSP α-chains and β-chains is required for RON activation.
Yokoyama, N., Ischenko, I., Hayman, M. J. & Miller, W. T. The C terminus of RON tyrosine kinase plays an autoinhibitory role. J. Biol. Chem. 280, 8893–8900 (2005).
Carafoli, F., Chirgadze, D. Y., Blundell, T. L. & Gherardi, E. Crystal structure of the β-chain of human hepatocyte growth factor-like/macrophage stimulating protein. FEBS J. 272, 5799–5807 (2005). This paper describes the crystal structure of the MSP β-chain and shows that three residues in the central cleft are essential for binding of MSP to RON. Moreover, the intermolecular interactions among the MSP β-chain did not create a receptor-contacting interface, suggesting that dimerization of the MSP β-chain is not required for RON activation.
Wang, J. et al. The crystal structure of a constitutively active mutant RON kinase suggests an intramolecular autophosphorylation hypothesis. Biochemistry 49, 7972–7974 (2010).
Willett, C. G. et al. Differential screening of a human chromosome 3 library identifies hepatocyte growth factor-like/macrophage-stimulating protein and its receptor in injured lung. Possible implications for neuroendocrine cell survival. J. Clin. Invest. 99, 2979–2991 (1997).
Ueda, A. & Yoshimura, T. Characterization of cis-acting elements of the gene for macrophage-stimulating protein from the human. The involvement of positive and negative regulatory elements. J. Biol. Chem. 271, 20265–20272 (1996).
Waltz, S. E., Gould, F. K., Air, E. L., McDowell, S. A. & Degen, S. J. Hepatocyte nuclear factor-4 is responsible for the liver-specific expression of the gene coding for hepatocyte growth factor-like protein. J. Biol. Chem. 271, 9024–9032 (1996).
Ueda, A., Takeshita, F., Yamashiro, S. & Yoshimura, T. Positive regulation of the human macrophage stimulating protein gene transcription. Identification of a new hepatocyte nuclear factor-4 (HNF-4) binding element and evidence that indicates direct association between NF-Y and HNF-4. J. Biol. Chem. 273, 19339–19347 (1998).
Muraoka, R. S., Waltz, S. E. & Degen, S. J. Expression of hepatocyte growth factor-like protein is repressed by retinoic acid and enhanced by cyclic adenosine 3′,5′-monophosphate response element-binding protein (CREB)-binding protein (CBP). Endocrinology 140, 187–196 (1999).
Wang, M. H., Skeel, A. & Leonard, E. J. Proteolytic cleavage and activation of pro-macrophage-stimulating protein by resident peritoneal macrophage membrane proteases. J. Clin. Invest. 97, 720–727 (1996).
Orikawa, H. et al. Activation of macrophage-stimulating protein by human airway trypsin-like protease. FEBS Lett. 586, 217–221 (2012).
Bhatt, A. S. et al. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc. Natl Acad. Sci. USA 104, 5771–5776 (2007).
Del Gatto, F., Gilbert, E., Ronsin, C. & Breathnach, R. Structure of the promoter for the human macrophage stimulating protein receptor gene. Biochim. Biophys. Acta 1263, 93–95 (1995).
Angeloni, D. et al. Gene structure of the human receptor tyrosine kinase RON and mutation analysis in lung cancer samples. Genes Chromosomes Cancer 29, 147–156 (2000).
Thangasamy, A., Rogge, J. & Ammanamanchi, S. Regulation of RON tyrosine kinase-mediated invasion of breast cancer cells. J. Biol. Chem. 283, 5335–5343 (2008).
Thangasamy, A., Rogge, J. & Ammanamanchi, S. Recepteur d'origine nantais tyrosine kinase is a direct target of hypoxia-inducible factor-1α-mediated invasion of breast carcinoma cells. J. Biol. Chem. 284, 14001–14010 (2009).
Zhao, S. et al. Smad4-dependent TGF-β signaling suppresses RON receptor tyrosine kinase-dependent motility and invasion of pancreatic cancer cells. J. Biol. Chem. 283, 11293–11301 (2008).
Thangasamy, A., Rogge, J., Krishnegowda, N. K., Freeman, J. W. & Ammanamanchi, S. Novel function of transcription factor Nrf2 as an inhibitor of RON tyrosine kinase receptor-mediated cancer cell invasion. J. Biol. Chem. 286, 32115–28622 (2011).
Lee, K. E. et al. Up-regulation of recepteur d'origine nantais tyrosine kinase and cell invasiveness via early growth response-1 in gastric cancer cells. J. Cell. Biochem. 113, 1217–1223 (2012).
Angeloni, D. et al. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene 26, 4499–4512 (2007).
Lu, Y., Yao, H. P. & Wang, M. H. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 257, –164 (2007).
Wang, M. H., Lao, W. F., Wang, D., Luo, Y. L. & Yao, H. P. Blocking tumorigenic activities of colorectal cancer cells by a splicing RON receptor variant defective in the tyrosine kinase domain. Cancer Biol. Ther. 6, 1121–1129 (2007).
Zhang, K., Zhou, Y. Q., Yao, H. P. & Wang, M. H. Alterations in a defined extracellular region of the RON receptor tyrosine kinase promote RON-mediated motile and invasive phenotypes in epithelial cells. Int. J. Oncol. 36, 255–264 (2010).
Ma, Q. et al. Inhibition of MSP-RON signaling pathway in cancer cells by a novel soluble form of RON comprising the entire sema sequence. Int. J. Oncol. 36, 1551–1561 (2010).
Angeloni, D., Danilkovitch-Miagkova, A., Miagkov, A., Leonard, E. J. & Lerman, M. I. The soluble sema domain of the RON receptor inhibits macrophage-stimulating protein-induced receptor activation. J. Biol. Chem. 279, 3726–3732 (2004).
Ghigna, C. et al. Pro-metastatic splicing of Ron proto-oncogene mRNA can be reversed: therapeutic potential of bifunctional oligonucleotides and indole derivatives. RNA Biol. 7, 495–503 (2010).
Chao, K. L., Tsai, I. W., Chen, C. & Herzberg, O. Crystal structure of the Sema-PSI extracellular domain of human RON receptor tyrosine kinase. PLoS ONE 7, e41912 (2012). This article describes the crystal structure of the RON SEMA–PSI domain, which shows that an interface is formed by a homodimer of the RON SEMA domains, which overlaps with the putative binding site in the MSP β-chain. The finding supports the model of one MSP molecule binding to two RON proteins leading to receptor dimerization and signalling activation.
Ponzetto, C. et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261–271 (1994).
Iwama, A., Yamaguchi, N. & Suda, T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 15, 5866–5875 (1996).
Xiao, Z. Q., Chen, Y. Q. & Wang, M. H. Requirement of both tyrosine residues 1330 and 1337 in the C-terminal tail of the RON receptor tyrosine kinase for epithelial cell scattering and migration. Biochem. Biophys. Res. Commun. 267, 669–675 (2000).
Santoro, M. M. et al. Point mutations in the tyrosine kinase domain release the oncogenic and metastatic potential of the Ron receptor. Oncogene 17, 741–749 (1998).
Chaudhuri, A. et al. Distinct involvement of the Gab1 and Grb2 adaptor proteins in signal transduction by the related receptor tyrosine kinases RON and MET. J. Biol. Chem. 286, 32762–33274 (2011).
Wang, M. H., Montero-Julian, F. A., Dauny, I. & Leonard, E. J. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene 13, 2167–2175 (1996).
Danilkovitch-Miagkova, A. et al. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the β-catenin pathway. Mol. Cell. Biol. 21, 5857–5868 (2001).
Park, J. S., Park, J. H., Khoi, P. N., Joo, Y. E. & Jung, Y. D. MSP-induced RON activation upregulates uPAR expression and cell invasiveness via MAPK, AP-1 and NF-κB signals in gastric cancer cells. Carcinogenesis 32, 175–181 (2011).
Danilkovitch, A., Donley, S., Skeel, A. & Leonard, E. J. Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells. Mol. Cell. Biol. 20, 2218–2227 (2000).
Santoro, M. M., Gaudino, G. & Marchisio, P. C. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell 5, 257–271.
Wang, M. H. et al. Macrophage-stimulating protein induces proliferation and migration of murine keratinocytes. Exp. Cell Res. 226, 39–46 (1996).
Côté, M., Miller, A. D. & Liu, S. L. Human RON receptor tyrosine kinase induces complete epithelial-to-mesenchymal transition but causes cellular senescence. Biochem. Biophys. Res. Commun. 360, 219–225 (2007).
Yu, P. T. et al. The RON-receptor regulates pancreatic cancer cell migration through phosphorylation-dependent breakdown of the hemidesmosome. Int. J. Cancer 131, 1744–1754 (2012).
Li, B. Q. et al. Macrophage-stimulating protein activates Ras by both activation and translocation of SOS nucleotide exchange factor. Biochem. Biophys. Res. Commun. 216, 110–118 (1995).
Eyob, H. et al. Inhibition of Ron kinase blocks conversion of micrometastases to overt metastases by boosting anti-tumor immunity. Cancer Discov. 23 Apr 2013 (doi:10.1158/2159-8290.CD-12-0480).
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. Met, metastasis, motility and more. Nature Rev. Mol. Cell. Biol. 4, 915–925 (2003).
Xu, X. M., Zhou, Y. Q. & Wang, M. H. Mechanisms of cytoplasmic β-catenin accumulation and its involvement in tumorigenic activities mediated by oncogenic splicing variant of the receptor originated from Nantes tyrosine kinase. J. Biol. Chem. 280, 25087–25094 (2005).
Zhou, Y. Q., Luo, Y. L., Zhou, Z. W., Yao, H. P. & Wang, M. H. β-Arrestin-1-dependent activation of extracellular signal-regulated kinases in RON receptor-mediated tumorigenic activities in colon cancer cells. J. Cancer Mol. 5, 55–62 (2010).
Danilkovitch-Miagkova, A. et al. Integrin-mediated RON growth factor receptor phosphorylation requires tyrosine kinase activity of both the receptor and c-Src. J. Biol. Chem. 275, 14783–14786 (2000).
Penengo, L., Rubin, C., Yarden, Y. & Gaudino, G. c-Cbl is a critical modulator of the Ron tyrosine kinase receptor. Oncogene 22, 3669–3679 (2003).
Germano, S. et al. Geldanamycins trigger a novel Ron degradative pathway, hampering oncogenic signaling. J. Biol. Chem. 281, 21710–21719 (2006).
Santoro, M. M., Gaudino, G. & Villa-Moruzzi, E. Protein phosphatase 1 binds to phospho-Ser-1394 of the macrophage-stimulating protein receptor. Biochem. J. 376, 587–594 (2003).
Ma, Q. et al. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol. Cancer 10, 66–75 (2010).
Doehn, U. et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol. Cell 35, 511–522 (2009).
Smolen, G. A. et al. A genome-wide RNAi screen identifies multiple RSK-dependent regulators of cell migration. Genes Dev. 24, 2654–2665 (2010).
Gurusamy, D. et al. Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth. Cancer Res. 73, 1752–1763 (2013).
Sharda, D. R. et al. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J. Immunol. 187, 2181–2192 (2011).
Follenzi, A. et al. Cross-talk between the proto-oncogenes Met and Ron. Oncogene 19, 3041–3049 (2000).
Benvenuti, S. et al. Ron kinase transphosphorylation sustains MET oncogene addiction. Cancer Res. 71, 1945–1955 (2011).
Peace, B. E., Hill, K. J., Degen, S. J. & Waltz, S. E. Cross-talk between the receptor tyrosine kinases Ron and epidermal growth factor receptor. Exp. Cell Res. 289, 317–225 (2003).
Thomas, C. Y. & Theodorescu, D. Collaboration of RON and epidermal growth factor receptor in human bladder carcinogenesis. J. Urol. 176, 1909–1910 (2006).
Liu, H. S. et al. An unusual function of RON receptor tyrosine kinase as a transcriptional regulator in cooperation with EGFR in human cancer cells. Carcinogenesis 31, 1456–1464 (2010). This paper reports that RON translocates from the cell membrane into the nucleus under stress conditions and that nuclear RON acts as a transcriptional factor that regulates expression of various genes.
Kobayashi, T. et al. Transactivation of RON receptor tyrosine kinase by interaction with PDGF receptor β during steady-state growth of human mesangial cells. Kidney Int. 75, 1173–1183 (2009).
Potratz, J. C. et al. Synthetic lethality screens reveal RPS6 and MST1R as modifiers of insulin-like growth factor-1 receptor inhibitor activity in childhood sarcomas. Cancer Res. 70, 8770–8781 (2010). This paper describes RON signalling acting as a compensatory pathway for tumour cell growth and survival in sarcoma cells in which IGF1R is inhibited.
Jaquish, D. V. et al. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis 32, 1151–1156 (2011).
Persons, D. A. et al. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nature Genet. 23, 159–165 (1999).
Nishigaki, K., Thompson, D., Hanson, C., Yugawa, T. & Ruscetti, S. The envelope glycoprotein of friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk. J. Virol. 75, 7893–7903 (2001). References 128 and 129 describe the role of mouse SF-RON in FLV-induced and erythropoietin-independent proliferation of erythroid cells, which leads to erythroid leukaemia in mice. This effect is mediated by the covalent interaction of the FLV envelope protein with mouse SF-RON.
Danilkovitch-Miagkova, A. et al. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl Acad. Sci. USA 100, 4580–4585 (2003).
Varela, M., Chow, Y. H., Sturkie, C., Murcia, P. & Palmarini, M. Association of RON tyrosine kinase with the Jaagsiekte sheep retrovirus envelope glycoprotein. Virology 350, 347–357 (2006).
Chou, Y. C. et al. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. Blood 118, 1340–1349 (2011).
Wagh, P. K. et al. β-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene 30, 3694–3704 (2011).
Wagh, P. K., Zinser, G. M., Gray, J. K., Shrestha, A. & Waltz, S. E. Conditional deletion of β-catenin in mammary epithelial cells of Ron receptor, Mst1r, overexpressing mice alters mammary tumorigenesis. Endocrinology 153, 2735–2746 (2012).
Peace, B. E., Toney-Earley, K., Collins, M. H. & Waltz, S. E. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 65, 1285–1293 (2005).
He, S. et al. Activation of the N-terminally truncated form of the Stk receptor tyrosine kinase Sf-Stk by Friend virus-encoded gp55 is mediated by cysteine residues in the ecotropic domain of gp55 and the extracellular domain of Sf-Stk. J. Virol. 84, 2223–2235 (2010).
Xu, X. M., Wang, D., Shen, Q., Chen, Y. Q. & Wang, M. H. RNA-mediated gene silencing of the RON receptor tyrosine kinase alters oncogenic phenotypes of human colorectal carcinoma cells. Oncogene 23, 8464–8474 (2004). This paper describes that knockdown of RON expression by siRNA in vitro inhibits growth and increases apoptotic death of tumour cells. These data indicate that certain cancer cells are dependent on RON signalling for growth and survival.
Zou, H. Y. et al. Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Mol. Cancer Ther. 11, 1036–1047 (2012).
Christensen, J. G. et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 63, 7345–7355 (2003).
Yao, H. P. et al. Oncogenic variant RON160 expression in breast cancer and its potential as a therapeutic target by small molecule tyrosine kinase inhibitor. Curr. Cancer Drug Target 17 Apr 2013 [epub ahead of print].
Zhang, Y. et al. Identification of a novel recepteur d'origine nantais/c-met small-molecule kinase inhibitor with antitumor activity in vivo. Cancer Res. 68, 6680–6687 (2008).
Schroeder, G. M. et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl) -4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J. Med. Chem. 52, 1251–1254 (2009).
Sharma, S. et al. Small molecule inhibitor BMS-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Mol. Cancer Ther. 12, 725–736 (2013).
O'Toole, J. M. et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 66, 9162–9170 (2006).
Yao, H. P. et al. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. Mol. Cancer 10, 82–91 (2011). References 144 and 145 describe the therapeutic effects of two RON-specific monoclonal antibodies in mouse tumour xenograft models and demonstrate that inhibition of RON signalling alone is sufficient to attenuate tumour growth.
Lee, W. Y. et al. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin. Cancer Res. 11, 2222–2228 (2005).
Cheng, H. L. et al. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. Br. J. Cancer. 92, 1906–1914 (2005).
Compérat, E. et al. Prognostic value of MET, RON and histoprognostic factors for urothelial carcinoma in the upper urinary tract. J. Urol. 179, 868–872 (2008).
Lee, C. T. et al. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis. Colon Rectum. 51, 1268–1274 (2008).
Ferrandina, G. et al. Prognostic role of the recepteur d'origine nantais (RON) expression in ovarian cancer patients. Gynecol. Oncol. 111, 237–243 (2008).
Zhou, D. et al. Expression of the RON receptor tyrosine kinase and its association with gastric carcinoma versus normal gastric tissues. BMC Cancer 8, 353–359 (2008).
Tactacan, C. M. et al. RON is not a prognostic marker for resectable pancreatic cancer. BMC Cancer 12, 395–403 (2012).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nature Genet. 16, 68–73 (1997).
Ma, P. C. et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer 47, 1025–1037 (2008).
Hui, M. K. et al. Prognostic significance of phosphorylated RON in esophageal squamous cell carcinoma. Med. Oncol. 29, 1699–1706 (2012).
Yoon, T. M. et al. Expression of the receptor tyrosine kinase recepteur d'origine nantais and its association with tumor progression in hypopharyngeal cancer. Head Neck 6 Aug 2013 (doi:10.1002/hed.23090).
Li, Z. et al. Monoclonal antibody (mAb)-induced down-regulation of RON receptor tyrosine kinase diminishes tumorigenic activities of colon cancer cells. Int. J. Oncol. 37, 473–482 (2010).
Gunes, Z. et al. Isolation of fully human antagonistic RON Antibodies showing efficient block of downstream signaling and cell migration. Transl. Oncol. 4, 38–46 (2011).
Guin, S., Yao, H. P. & Wang, M. H. RON receptor tyrosine kinase as a target for delivery of chemodrugs by antibody directed pathway for cancer cell cytotoxicity. Mol. Pharmaceutics. 7, 386–397 (2010).
Guin, S. et al. Targeting acute hypoxic cancer cells by doxorubicin-immunoliposomes directed by monoclonal antibodies specific to RON receptor tyrosine kinase. Cancer Chemother. Pharmacol. 67, 1073–1083 (2011).
Padhye, S. S. et al. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol. Pharm. 8, 2310–2319 (2011).
Sievers, E. L. & Senter, P. D. Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 64, 15–29 (2013). References 160–162 describe RON as a targeting moiety for antibody-directed delivery of chemotherapeutic agents for enhanced cancer cell cytotoxicity. The proof-of-concept provides the rationale for the development of RON ADCs for potential clinical application.
Verma, S. et al. EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Katz, J., Janik, J. E. & Younes, A. Brentuximab Vedotin (SGN-35). Clin. Cancer Res. 17, 6428–6436 (2011).
Moldenhauer, G. et al. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl Cancer Inst. 104, 622–634 (2012).
Narasimhan, M. & Ammanamanchi, S. Curcumin blocks RON tyrosine kinase-mediated invasion of breast carcinoma cells. Cancer Res. 68, 5185–5192 (2008).
Ni, S., Zhao, C., Feng, G. S., Paulson, R. F. & Correll, P. H. A novel Stat3 binding motif in Gab2 mediates transformation of primary hematopoietic cells by the Stk/Ron receptor tyrosine kinase in response to Friend virus infection. Mol. Cell. Biol. 27, 3708–3715 (2007).
Umehara, D. et al. Role of phosphatidylinositol 3-kinase in friend spleen focus-forming virus-induced erythroid disease. J. Virol. 84, 7675–7682 (2010).
Choueiri, T. K. et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 31, 181–186 (2013).
Qian, F. et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 69, 8009–8016 (2009).
Eder, J. P. et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin. Cancer Res. 16, 3507–3516 (2010).
Belalcazar, A., Azaña, D., Perez, C. A., Raez, L. E. & Santos, E. S. Targeting the Met pathway in lung cancer. Expert Rev. Anticancer Ther. 12, 519–528 (2012).
Katz, J. D. et al. Discovery of a 5H-benzo[4,5]cyclohepta[1,2-b]pyridin-5-one (MK-2461) inhibitor of c-Met kinase for the treatment of cancer. J. Med. Chem. 54, 4092–4108 (2011).
Pan, B. S. et al. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 70, 1524–1533 (2010).
Northrup, A. B. et al. Discovery of 1-[3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo [4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesul-fonamide (MK-8033): a specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J. Med. Chem. 56, 2294–2310 (2013).
Acknowledgements
This work was supported in part by US National Institutes of Health grant R01 CA91980 (M.H.W.) and a grant from the Amarillo Area Foundation (M.H.W.). R.W.Z. was supported by NIH grants R01 CA112029 and CA121211. Support was also provided by Research Subproject #2011ZZ01 to M.H.W. from State Key Laboratory for Diagnosis & Treatment of Infectious Diseases in First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, P. R. China. The assistance of S. Denney (Texas Tech University Health Sciences Center School of Pharmacy, Amarillo, Texas, USA) in editing the manuscript is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Protein truncation
-
A biochemical process by which a portion of amino acid residues is removed from either the amino or the carboxyl terminus, resulting in a protein isoform.
- Multifunctional docking site
-
A tandemly arranged sequence in the carboxy-terminal tail of MET and RON that recruits multiple SH2-containing molecules such as PI3K.
- Epithelial-to-mesenchymal transition
-
(EMT). A phenotypic change of epithelial cells characterized by spindle cell morphology, loss of epithelial properties and gain of mesenchymal markers with increased cell motility.
- Kringle domains
-
Protein structures formed roughly by 80 amino acids that fold into a triple disulphide-linked loop resembling the shape of a Scandinavian pastry known as kringle.
- Friend leukaemia virus
-
(FLV). A strain of mouse retrovirus also known as Friend spleen focus-forming virus that infects mouse erythroid cells and subsequently causes erythroleukaemia.
- Jaagsiekte sheep retrovirus
-
(JSRV). A strain of retrovirus that is the causative agent of a contagious lung cancer called ovine pulmonary adenocarcinoma in sheep.
- Epstein–Barr virus
-
(EBV). A herpes virus that causes infectious mononucleosis, transforms B lymphocytes and is associated with lymphoid malignancies such as Burkett's lymphoma.
- Signalling-compensatory mechanism
-
A biochemical event in which an alternative signalling pathway is activated, allowing continued cell growth and survival when other pathways are inhibited or disrupted by molecularly targeted therapeutics.
- Hemidesmosomes
-
Small rivet-like structures on the inner basal surface of epithelial cells, which attach one cell to the extracellular matrix for cell adhesion.
- Polycythemia
-
An abnormal status in which the concentration of red cells increases as a percentage of total blood volume owing to reduced plasma volume or intrinsic growth of the myeloid stem cells.
Rights and permissions
About this article
Cite this article
Yao, HP., Zhou, YQ., Zhang, R. et al. MSP–RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer 13, 466–481 (2013). https://doi.org/10.1038/nrc3545
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3545
This article is cited by
-
MAGOH promotes gastric cancer progression via hnRNPA1 expression inhibition-mediated RONΔ160/PI3K/AKT signaling pathway activation
Journal of Experimental & Clinical Cancer Research (2024)
-
A novel anoikis-related gene signature identifies LYPD1 as a novel therapy target for bladder cancer
Scientific Reports (2024)
-
Targeting isoforms of RON kinase (MST1R) drives antitumor efficacy
Cell Death & Differentiation (2023)
-
A network map of macrophage-stimulating protein (MSP) signaling
Journal of Cell Communication and Signaling (2023)
-
Role of plectin and its interacting molecules in cancer
Medical Oncology (2023)