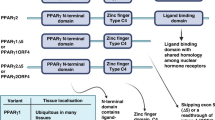
Key Points
-
Peroxisome proliferator-activated receptors (PPARs) have central roles in the regulation of glucose and lipid homeostasis through their functions as molecular sensors that respond to endogenous ligands, leading to the modulation of gene expression. PPARs also regulate cell proliferation, differentiation and inflammation.
-
PPARα mediates hepatocarcinogenesis induced by long-term administration of PPARα agonists in rodent models, an effect that is not found in humans. The mechanism underlying species-specific hepatocarcinogenesis is through mouse PPARα-dependent regulation of the let-7c microRNA, which leads to increased expression of the oncoprotein MYC. The current interest in targeting PPARα for the prevention of certain cancers, including colon and leukaemia, is based on studies showing that PPARα agonists inhibit the proliferation of endothelial cells, increase the synthesis of PPARγ agonists and potentially interfere with the Warburg effect.
-
The role of PPARβ/δ in carcinogenesis is controversial. Several studies have shown that PPARβ/δ is upregulated in cancer cells by the adenomatous polyposis coli (APC)–β-catenin–TCF4 pathway and has a pro-tumorigenic effect in many cancer types. However, other studies have shown that PPARβ/δ agonists can induce terminal differentiation and inhibit innate inflammation, suggesting anticancer effects. In addition, a retrospective study has shown that low expression levels of PPARβ/δ are associated with the decreased survival of patients with colorectal cancer. Therefore, there remains a need to further examine PPARβ/δ protein expression patterns quantitatively in tumour models and the putative mechanisms that are mediated by PPARβ/δ agonists associated with anti-apoptotic or growth stimulatory effects.
-
PPARγ agonists can induce terminal differentiation, inhibit cell proliferation, promote apoptosis and inhibit innate inflammation in many cancer models. This has led to a number of clinical trials with PPARγ agonists, but these have generated mixed results. Moreover, some PPARγ agonists have been associated with pro-tumorigenic effects. Emerging evidence indicates that targeting PPARγ in combination with other chemopreventive or chemotherapeutic agents might increase the efficacy of the effects that are induced by monotherapies.
-
Owing to similarities in the abilities of the three PPARs to improve different metabolic disorders that are known to be associated with increased cancer risk (such as diabetes, obesity, dyslipidemias and chronic inflammation), modulating the activities of the PPARs remains an attractive approach for the treatment and prevention of cancer. The challenge is to advance the discovery of molecular mechanisms of action in order to identify and characterize effective PPAR agonists with acceptable safety profiles.
Abstract
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that are involved in regulating glucose and lipid homeostasis, inflammation, proliferation and differentiation. Although all of these functions might contribute to the influence of PPARs in carcinogenesis, there is a distinct need for a review of the literature and additional experimentation to determine the potential for targeting PPARs for cancer therapy and cancer chemoprevention. As PPAR agonists include drugs that are used for the treatment of metabolic diseases, a more complete understanding of the roles of PPARs in cancer will aid in determining any increased cancer risk for patients undergoing therapy with PPAR agonists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schupp, M. & Lazar, M. A. Endogenous ligands for nuclear receptors: digging deeper. J. Biol. Chem. 285, 40409–40415 (2010).
Shi, Y., Hon, M. & Evans, R. M. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proc. Natl Acad. Sci. USA 99, 2613–2618 (2002).
Adhikary, T. et al. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-β/δ (PPARβ/δ). PLoS ONE 6, e16344 (2011).
Borland, M. G. et al. Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes. Cell. Signal. 23, 2039–2050 (2011). This study critically examined possible mechanisms of PPARβ/δ-dependent regulation, including whether retinoic acid activates PPARβ/δ, whether PPARβ/δ represses PPARγ activity and how PPARβ/δ regulates apoptosis and inflammatory cytokine expression following exposure to ultraviolet light.
Marin, H. E. et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits colon carcinogenesis. Cancer Res. 66, 4394–4401 (2006).
Matsusue, K., Peters, J. M. & Gonzalez, F. J. PPARβ/δ potentiates PPARγ-stimulated adipocyte differentiation. FASEB J. 18, 1477–1479 (2004).
Peters, J. M., Aoyama, T., Burns, A. M. & Gonzalez, F. J. Bezafibrate is a dual ligand for PPARα and PPARβ: studies using null mice. Biochim. Biophys. Acta 1632, 80–89 (2003).
Kilgore, K. S. & Billin, A. N. PPARβ/δ ligands as modulators of the inflammatory response. Curr. Opin. Investig. Drugs 9, 463–469 (2008).
Peters, J. M., Foreman, J. E. & Gonzalez, F. J. Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast and lung carcinogenesis. Cancer Metastasis Rev. 30, 619–640 (2011).
Peters, J. M. & Gonzalez, F. J. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim. Biophys. Acta 1796, 230–241 (2009).
Peters, J. M., Hollingshead, H. E. & Gonzalez, F. J. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin. Sci. 115, 107–127 (2008).
Peters, J. M., Morales, J. L. & Gonzales, F. J. Modulation of gastrointestinal inflammation and colorectal tumorigenesis by peroxisome proliferator-activated receptor-β/δ (PPARβ/δ). Drug Discov. Today Dis. Mech. 29 Nov 2011 (doi: 10.1016/j.ddmec.2011.11.002).
Varga, T., Czimmerer, Z. & Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 1812, 1007–1022 (2011).
Pascual, G. et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 437, 759–763 (2005).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007). A good review of the literature describing mechanisms of trans-repression by PPARs.
Choi, J. H. et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 477, 477–481 (2011). This study demonstrated the feasibility of targeting PPARγ with a non-agonist to elicit anti-diabetic activity without causing the negative side effects that are associated with some PPARγ agonists.
Issemann, I. & Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347, 645–650 (1990). This study describes the cloning and characterization of PPARα, the first of the three PPARs to be identified.
Escher, P. et al. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 142, 4195–4202 (2001).
Pyper, S. R., Viswakarma, N., Yu, S. & Reddy, J. K. PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept Signal. 8, e002 (2010).
Mandard, S., Muller, M. & Kersten, S. Peroxisome proliferator-activated receptor α target genes. Cell. Mol. Life Sci. 61, 393–416 (2004). A useful resource listing PPARα target genes and corresponding references.
Kersten, S. et al. The peroxisome proliferator-activated receptor α regulates amino acid metabolism. FASEB J. 15, 1971–1978 (2001).
Guerre-Millo, M. et al. Peroxisome Proliferator-activated Receptorα activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 275, 16638–16642 (2000).
Uhlen, M. et al. Towards a knowledge-based Human Protein Atlas. Nature Biotech. 28, 1248–1250 (2010).
Girroir, E. E. et al. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem. Biophys. Res. Commun. 371, 456–461 (2008).
Leibowitz, M. D. et al. Activation of PPARδ alters lipid metabolism in db/db mice. FEBS Lett. 473, 333–336 (2000). This study was one of the first to demonstrate a functional phenotype resulting from activating PPARβ/δ.
Oliver, W. R. Jr. et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc. Natl Acad. Sci. USA 98, 5306–5311 (2001).
Sprecher, D. L. et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist. Arterioscler. Thromb. Vasc. Biol. 27, 359–365 (2007).
Tanaka, T. et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl Acad. Sci. USA 100, 15924–15929 (2003).
Wang, Y. X. et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113, 159–170 (2003).
Wang, Y. X. et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2, e294 (2004).
Lim, H. J. et al. PPARδ ligand L-165041 ameliorates Western diet-induced hepatic lipid accumulation and inflammation in LDLR−/− mice. Eur. J. Pharmacol. 622, 45–51 (2009).
Liu, S. et al. Role of peroxisome proliferator-activated receptor δ/β in hepatic metabolic regulation. J. Biol. Chem. 286, 1237–1247 (2011).
Nagasawa, T. et al. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARδ agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur. J. Pharmacol. 536, 182–191 (2006).
Shan, W. et al. Peroxisome proliferator-activated receptor-β/δ protects against chemically induced liver toxicity in mice. Hepatology 47, 225–235 (2008).
Shan, W. et al. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol. Sci. 105, 418–428 (2008).
Burdick, A. D., Kim, D. J., Peraza, M. A., Gonzalez, F. J. & Peters, J. M. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell. Signal. 18, 9–20 (2006).
Zhu, Y. et al. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proc. Natl Acad. Sci. USA 92, 7921–7925 (1995).
Fajas, L., Fruchart, J. C. & Auwerx, J. PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Lett. 438, 55–60 (1998).
Foreman, J. E. et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol. Carcinog. 48, 942–952 (2009); erratum 50, 652–653 (2011).
Abbott, B. D., Wood, C. R., Watkins, A. M., Das, K. P. & Lau, C. S. Peroxisome proliferator-activated receptors α, β, and γ mRNA and protein expression in human fetal tissues. PPAR Res. 2010, 690907 (2010); erratum 2010, 627284 (2010).
Barak, Y. et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999).
Tontonoz, P., Hu, E., Graves, R. A., Budavari, A. I. & Spiegelman, B. M. mPPAR γ 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 (1994).
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994).
Semple, R. K., Chatterjee, V. K. & O'Rahilly, S. PPAR γ and human metabolic disease. J. Clin. Invest. 116, 581–589 (2006).
Reddy, J. K., Azarnoff, D. L. & Hignite, C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 283, 397–398 (1980). This study was one of the first to demonstrate that long-term administration of PPARα agonists causes liver cancer in rodents.
Peters, J. M., Cattley, R. C. & Gonzalez, F. J. Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis 18, 2029–2033 (1997). This study established that PPARα is required to mediate hepatocarcinogenesis caused by long-term administration of PPARα agonists in mice.
Hays, T. et al. Role of peroxisome proliferator-activated receptor-α (PPARα) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 26, 219–227 (2005).
Peters, J. M., Cheung, C. & Gonzalez, F. J. Peroxisome proliferator-activated receptor-α and liver cancer: where do we stand? J. Mol. Med. 83, 774–785 (2005).
Cheung, C. et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor-α. Cancer Res. 64, 3849–3854 (2004).
Morimura, K., Cheung, C., Ward, J. M., Reddy, J. K. & Gonzalez, F. J. Differential susceptibility of mice humanized for peroxisome proliferator-activated receptor α to Wy-14,643-induced liver tumorigenesis. Carcinogenesis 27, 1074–1080 (2006). This study demonstrated that PPARα-humanized transgenic models do not develop liver tumours after long-term administration of PPARα agonists, suggesting a species difference in activities between human and rodent PPARα.
Shah, Y. M. et al. Peroxisome proliferator-activated receptor α regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol. Cell. Biol. 27, 4238–4247 (2007). This study helped to elucidate the mechanism that explains why human PPARα does not mediate hepatocarcinogenesis, but the mouse PPARα does, by showing differential regulation of let-7c miRNA.
He, T. C., Chan, T. A., Vogelstein, B. & Kinzler, K. W. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99, 335–345 (1999).
Modica, S. et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology 138, 636–648 (2010).
Foreman, J. E. et al. Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol. Carcinog. 50, 884–900 (2011). This is the most quantitative study to date showing that expression of PPARβ/δ protein is lower in human and rodent colon tumours compared with non-transformed tissue and also includes functional characterization of overexpression of PPARβ/δ in human colon cancer cell lines.
Delage, B., Rullier, A., Capdepont, M., Rullier, E. & Cassand, P. The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers. Nutr. J. 6, 20 (2007).
Gupta, R. A. et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proc. Natl Acad. Sci. USA 97, 13275–13280 (2000).
Roy, H. K., Karolski, W. J. & Ratashak, A. Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int. J. Cancer 92, 609–615 (2001).
Takayama, O. et al. Expression of PPARδ in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. Br. J. Cancer 95, 889–895 (2006).
Wang, D., Ning, W., Xie, D., Guo, L. & Dubois, R. N. Peroxisome proliferator-activated receptor δ confers resistance to peroxisome proliferator-activated receptor γ-induced apoptosis in colorectal cancer cells. Oncogene 18 July 2011 (doi: 10.1038/onc.2011.299).
Yang, L. et al. Biological function and prognostic significance of peroxisome proliferator-activated receptor δ in rectal cancer. Clin. Cancer Res. 17, 3760–3770 (2011). This study provides the strongest clinical evidence to date showing that PPARβ/δ protects against colorectal cancer in humans.
Yoshinaga, M. et al. The simultaneous expression of peroxisome proliferator-activated receptor delta and cyclooxygenase-2 may enhance angiogenesis and tumor venous invasion in tissues of colorectal cancers. Dig. Dis. Sci. 54, 1108–1114 (2009).
Yoshinaga, M. et al. The expression of both peroxisome proliferator-activated receptor δ and cyclooxygenase-2 in tissues is associated with poor prognosis in colorectal cancer patients. Dig. Dis. Sci. 56, 1194–1200 (2011).
Davidson, B., Hadar, R., Stavnes, H. T., Trope, C. G. & Reich, R. Expression of the peroxisome proliferator-activated receptors-α, -β, and -γ in ovarian carcinoma effusions is associated with poor chemoresponse and shorter survival. Hum. Pathol. 40, 705–713 (2009).
Glazer, R. I., Yuan, H., Xie, Z. & Yin, Y. PPARγ and PPARδ as modulators of neoplasia and cell fate. PPAR Res. 2008, 247379 (2008).
Jaeckel, E. C. et al. Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor δ with head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 127, 1253–1259 (2001).
Nijsten, T., Geluyckens, E., Colpaert, C. & Lambert, J. Peroxisome proliferator-activated receptors in squamous cell carcinoma and its precursors. J. Cutan. Pathol. 32, 340–347 (2005).
Tong, B. J. et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator- activated receptor-δ in human endometrial adenocarcinoma. Neoplasia 2, 483–490 (2000).
Chen, L. C. et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 64, 3694–3700 (2004).
Hao, C. Y. et al. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin. Cancer Res. 11, 1400–1407 (2005).
Harman, F. S. et al. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nature Med. 10, 481–483 (2004).
Knutsen, H. K. et al. Increased levels of PPARβ/δ and cyclin D1 in flat dysplastic ACF and adenomas in ApcMin/+ mice. Anticancer Res. 25, 3781–3789 (2005).
Notterman, D. A., Alon, U., Sierk, A. J. & Levine, A. J. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 61, 3124–3130 (2001).
Orner, G. A. et al. Suppression of tumorigenesis in the Apcmin mouse: down-regulation of β-catenin signaling by a combination of tea plus sulindac. Carcinogenesis 24, 263–267 (2003).
Reed, K. R. et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene 23, 8992–8996 (2004).
Feilchenfeldt, J., Brundler, M. A., Soravia, C., Totsch, M. & Meier, C. A. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARγ-coactivator 1 (PGC-1). Cancer Lett. 203, 25–33 (2004).
Yang, L. et al. Quantitative analysis of PPARδ mRNA by real-time RT-PCR in 86 rectal cancer tissues. Eur. J. Surg. Oncol. 32, 181–185 (2006).
Ahmed, N., Riley, C. & Quinn, M. A. An immunohistochemical perspective of PPARβ and one of its putative targets PDK1 in normal ovaries, benign and malignant ovarian tumours. Br. J. Cancer 98, 1415–1424 (2008).
Yoshimura, R. et al. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int. J. Cancer 104, 597–602 (2003).
Foreman, J. E. et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol. Carcinog. 48, 942–952 (2009).
Ouyang, N., Williams, J. L. & Rigas, B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)δ expression in APCmin/+ mice proportionally to their tumor inhibitory effect: implications for the role of PPARδ in carcinogenesis. Carcinogenesis 27, 232–239 (2006).
Fevr, T., Robine, S., Louvard, D. & Huelsken, J. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 27, 7551–7559 (2007).
Sansom, O. J. et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18, 1385–1390 (2004).
Sheehan, K. M. et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 282, 1254–1257 (1999).
Hollingshead, H. E. et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and inhibition of cyclooxygenase 2 (COX2) attenuate colon carcinogenesis through independent signaling mechanisms. Carcinogenesis 29, 169–176 (2008).
Gupta, R. A. et al. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nature Med. 10, 245–247 (2004).
Wang, D. et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proc. Natl Acad. Sci. USA 103, 19069–19074 (2006).
Zuo, X. et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-δ and colonic tumorigenesis. J. Natl Cancer Inst. 101, 762–767 (2009).
Bility, M. T. et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits chemically-induced skin tumorigenesis. Carcinogenesis 29, 2406–2414 (2008).
Bility, M. T., Zhu, B., Kang, B. H., Gonzalez, F. J. & Peters, J. M. Ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase-2 enhances inhibition of skin tumorigenesis. Toxicol. Sci. 113, 27–36 (2010).
Kim, D. J. et al. Peroxisome proliferator-activated receptor β (δ)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J. Biol. Chem. 279, 23719–23727 (2004).
Kim, D. J., Prabhu, K. S., Gonzalez, F. J. & Peters, J. M. Inhibition of chemically-induced skin carcinogenicity by sulindac is independent of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ). Carcinogenesis 27, 1105–1112 (2006).
Zhu, B. et al. Chemoprevention of chemically induced skin tumorigenesis by ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase 2. Mol. Cancer Ther. 9, 3267–3277 (2011).
Di-Poi, N., Tan, N. S., Michalik, L., Wahli, W. & Desvergne, B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 10, 721–733 (2002).
Tan, N. S. et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes Dev. 15, 3263–3277 (2001).
Borland, M. G. et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell proliferation in human HaCaT keratinocytes. Mol. Pharmacol. 74, 1429–1442 (2008).
Burdick, A. D. et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cell. Signal. 19, 1163–1171 (2007).
Kim, D. J. et al. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 13, 53–60 (2006).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Pollock, C. B. et al. PPARδ activation acts cooperatively with 3-phosphoinositide-dependent protein kinase-1 to enhance mammary tumorigenesis. PLoS ONE 6, e16215 (2011).
Tachibana, K. et al. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nucl. Recept. 3, 3 (2005).
Szeles, L. et al. Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol. Endocrinol. 24, 2218–2231 (2010).
Hollingshead, H. E. et al. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis 28, 2641–2649 (2007).
Schug, T. T., Berry, D. C., Shaw, N. S., Travis, S. N. & Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129, 723–733 (2007).
Rohrl, C. et al. Peroxisome-proliferator-activated receptors γ and β/δ mediate vascular endothelial growth factor production in colorectal tumor cells. J. Cancer Res. Clin. Oncol. 137, 29–39 (2011).
Stephen, R. L. et al. Activation of peroxisome proliferator-activated receptor δ stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 64, 3162–3170 (2004).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Grommes, C., Landreth, G. E. & Heneka, M. T. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncol. 5, 419–429 (2004).
Koeffler, H. P. Peroxisome proliferator-activated receptor γ and cancers. Clin. Cancer Res. 9, 1–9 (2003).
Ogino, S. et al. Colorectal cancer expression of peroxisome proliferator-activated receptor γ (PPARG, PPARγ) is associated with good prognosis. Gastroenterology 136, 1242–1250 (2009). This study provided clinical evidence showing that PPARγ protects against colorectal cancer in humans.
McAlpine, C. A., Barak, Y., Matise, I. & Cormier, R. T. Intestinal-specific PPARγ deficiency enhances tumorigenesis in ApcMin/+ mice. Int. J. Cancer 119, 2339–2346 (2006).
Elnemr, A. et al. PPARγ ligand (thiazolidinedione) induces growth arrest and differentiation markers of human pancreatic cancer cells. Int. J. Oncol. 17, 1157–1164 (2000).
Gupta, R. A., Brockman, J. A., Sarraf, P., Willson, T. M. & DuBois, R. N. Target genes of peroxisome proliferator-activated receptor γ in colorectal cancer cells. J. Biol. Chem. 276, 29681–29687 (2001).
Sarraf, P. et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nature Med. 4, 1046–1052 (1998).
Tontonoz, P. et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc. Natl Acad. Sci. USA 94, 237–241 (1997). This study was one of the first to establish that PPARγ ligands can induce differentiation in human cancer cells providing support for the hypothesis that targeting PPARγ may be suitable for human cancers.
Yoshizumi, T. et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-γ ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int. J. Oncol. 25, 631–639 (2004).
Drori, S. et al. Hic-5 regulates an epithelial program mediated by PPARγ. Genes Dev. 19, 362–375 (2005).
Huang, J. W. et al. Peroxisome proliferator-activated receptor γ-independent ablation of cyclin D1 by thiazolidinediones and their derivatives in breast cancer cells. Mol. Pharmacol. 67, 1342–1348 (2005).
Lapillonne, H. et al. Activation of peroxisome proliferator-activated receptor γ by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 63, 5926–5939 (2003).
Qin, C. et al. Peroxisome proliferator-activated receptor γ agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor α in MCF-7 breast cancer cells. Cancer Res. 63, 958–964 (2003).
Wang, C. et al. Inhibition of cellular proliferation through IκB kinase-independent and peroxisome proliferator-activated receptor γ-dependent repression of cyclin D1. Mol. Cell. Biol. 21, 3057–3070 (2001).
Yin, F. et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem. Biophys. Res. Commun. 286, 916–922 (2001).
Koga, H. et al. Involvement of p21WAF1/Cip1, p27Kip1, and p18INK4c in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology 33, 1087–1097 (2001).
Chen, F. & Harrison, L. E. Ciglitazone-induced cellular anti-proliferation increases p27kip1 protein levels through both increased transcriptional activity and inhibition of proteasome degradation. Cell. Signal. 17, 809–816 (2005).
Chen, F., Kim, E., Wang, C. C. & Harrison, L. E. Ciglitazone-induced p27 gene transcriptional activity is mediated through Sp1 and is negatively regulated by the MAPK signaling pathway. Cell. Signal. 17, 1572–1577 (2005).
Itami, A. et al. Ligands for peroxisome proliferator-activated receptor γ inhibit growth of pancreatic cancers both in vitro and in vivo. Int. J. Cancer 94, 370–376 (2001).
Koga, H. et al. Troglitazone induces p27Kip1-associated cell-cycle arrest through down-regulating Skp2 in human hepatoma cells. Hepatology 37, 1086–1096 (2003).
Motomura, W., Okumura, T., Takahashi, N., Obara, T. & Kohgo, Y. Activation of peroxisome proliferator-activated receptor γ by troglitazone inhibits cell growth through the increase of p27KiP1 in human. Pancreatic carcinoma cells. Cancer Res. 60, 5558–5564 (2000).
Sharma, C., Pradeep, A., Wong, L., Rana, A. & Rana, B. Peroxisome proliferator-activated receptor γ activation can regulate β-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J. Biol. Chem. 279, 35583–35594 (2004).
Wei, S. et al. Thiazolidinediones modulate the expression of β-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor γ. Mol. Pharmacol. 72, 725–733 (2007).
Palakurthi, S. S., Aktas, H., Grubissich, L. M., Mortensen, R. M. & Halperin, J. A. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Res. 61, 6213–6218 (2001).
Bae, M. A. & Song, B. J. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol. Pharmacol. 63, 401–408 (2003).
Zander, T. et al. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARγ. J. Neurochem. 81, 1052–1060 (2002).
Shiau, C. W. et al. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARγ. Cancer Res. 65, 1561–1569 (2005).
Farrow, B. & Evers, B. M. Activation of PPARγ increases PTEN expression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 301, 50–53 (2003).
Lee, S. Y. et al. PPAR-γ agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer 51, 297–301 (2006).
Patel, L. et al. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr. Biol. 11, 764–768 (2001).
Teresi, R. E. et al. Increased PTEN expression due to transcriptional activation of PPARγ by Lovastatin and Rosiglitazone. Int. J. Cancer 118, 2390–2398 (2006).
Zhang, W. et al. PPARγ activator rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biol. Ther. 5, 1008–1014 (2006).
Kim, K. Y., Kim, S. S. & Cheon, H. G. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-γ agonists in MCF-7 breast cancer cells. Biochem. Pharmacol. 72, 530–540 (2006).
Yan, K. H. et al. The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol. Carcinog. 49, 235–246 (2010).
Kim, Y., Suh, N., Sporn, M. & Reed, J. C. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J. Biol. Chem. 277, 22320–22329 (2002).
Schultze, K. et al. Troglitazone sensitizes tumor cells to TRAIL-induced apoptosis via down-regulation of FLIP and Survivin. Apoptosis 11, 1503–1512 (2006).
Wei, S., Yang, J., Lee, S. L., Kulp, S. K. & Chen, C. S. PPARγ-independent antitumor effects of thiazolidinediones. Cancer Lett. 276, 119–124 (2009).
Glass, C. K. & Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature Rev. Immunol. 10, 365–376 (2010).
Adachi, M. et al. Peroxisome proliferator activated receptor γ in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 55, 1104–1113 (2006). This study demonstrated that PPARγ inhibits inflammation in the gut and protects against inflammatory bowel, which may explain in part the protective nature of PPARγ in colorectal cancer.
Shah, Y. M., Morimura, K. & Gonzalez, F. J. Expression of peroxisome proliferator-activated receptor-γ in macrophage suppresses experimentally induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G657–G666 (2007).
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Lefebvre, A. M. et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Med. 4, 1053–1057 (1998).
Saez, E. et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Med. 4, 1058–1061 (1998).
Pino, M. V., Kelley, M. F. & Jayyosi, Z. Promotion of colon tumors in C57BL/6J-APCmin/+ mice by thiazolidinedione PPARγ agonists and a structurally unrelated PPARγ agonist. Toxicol. Pathol. 32, 58–63 (2004).
Yang, K. et al. Peroxisome proliferator-activated receptor γ agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in Apc1638 N/+Mlh1+/− double mutant mice. Int. J. Cancer 116, 495–499 (2005).
Lubet, R. A. et al. Rosiglitazone, a PPARγ agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int. J. Cancer 123, 2254–2259 (2008).
Piccinni, C., Motola, D., Marchesini, G. & Poluzzi, E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 34, 1369–1371 (2011).
Fenner, M. H. & Elstner, E. Peroxisome proliferator-activated receptor-γ ligands for the treatment of breast cancer. Expert Opin. Investig. Drugs 14, 557–568 (2005).
Saez, E. et al. PPARγ signaling exacerbates mammary gland tumor development. Genes Dev. 18, 528–540 (2004).
Li, Y. & Lazar, M. A. Differential gene regulation by PPARγ agonist and constitutively active PPARγ2. Mol. Endocrinol. 16, 1040–1048 (2002).
Panigrahy, D. et al. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl Acad. Sci. USA 105, 985–990 (2008).
Pozzi, A. et al. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. J. Biol. Chem. 282, 17685–17695 (2007).
Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 25, 409–416 (2006).
Aoyama, A. et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J. Biol. Chem. 273, 5678–5684 (1998).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Rev. Cancer 11, 325–337 (2011).
Tsugane, S. & Inoue, M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 101, 1073–1079 (2010).
Wolin, K. Y., Carson, K. & Colditz, G. A. Obesity and cancer. Oncologist 15, 556–565 (2011).
Kasuga, J. et al. Novel biphenylcarboxylic acid peroxisome proliferator-activated receptor (PPAR) δ selective antagonists. Bioorg. Med. Chem. Lett. 19, 6595–6599 (2009).
Naruhn, S. et al. High affinity peroxisome proliferator-activated receptor β/δ-specific ligands with pure antagonistic or inverse agonistic properties. Mol. Pharmacol. 80, 828–838 (2011).
Shearer, B. G. et al. Identification and characterization of a selective peroxisome proliferator-activated receptor β/δ (NR1C2) antagonist. Mol. Endocrinol. 22, 523–529 (2008).
Shearer, B. G. et al. Identification and characterization of 4-chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787), a selective and irreversible peroxisome proliferator-activated receptor δ (PPARδ) antagonist. J. Med. Chem. 53, 1857–1861 (2010).
Zaveri, N. T. et al. A novel peroxisome proliferator-activated receptor δ antagonist, SR13904, has anti-proliferative activity in human cancer cells. Cancer Biol. Ther. 8, 1252–1261 (2009).
Palkar, P. S. et al. Cellular and pharmacological selectivity of the PPARβ/δ antagonist GSK3787. Mol. Pharmacol. 78, 419–430 (2010).
Demetri, G. D. & et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc. Natl Acad. Sci. USA 96, 3951–3956 (1999).
Debrock, G. et al. A phase II trial with rosiglitazone in liposarcoma patients. Br. J. Cancer 89, 1409–1412 (2003).
Smith, M. R. et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer 101, 1569–1574 (2004).
Hisatake, J. I. et al. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor γ in human prostate cancer. Cancer Res. 60, 5494–5498 (2000).
Mueller, E. et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc. Natl Acad. Sci. USA 97, 10990–10995 (2000).
Schwartz, G. K. et al. Phase I and pharmacokinetic study of LY293111, an orally bioavailable LTB4 receptor antagonist, in patients with advanced solid tumors. J. Clin. Oncol. 23, 5365–5373 (2005).
Burstein, H. J. et al. Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res. Treat. 79, 391–397 (2003).
Kulke, M. H. et al. A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J. 8, 395–399 (2002).
Baetz, T. et al. A phase I study of oral LY293111 given daily in combination with irinotecan in patients with solid tumours. Invest. New Drugs 25, 217–225 (2007).
Read, W. L., Baggstrom, M. Q., Fracasso, P. M. & Govindan, R. A phase I study of bexarotene and rosiglitazone in patients with refractory cancers. Chemotherapy 54, 236–241 (2008).
Hau, P. et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-γ agonists in recurrent high-grade gliomas - a phase II study. Oncology 73, 21–25 (2007).
Kebebew, E. et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery 140, 960–966; discussion 966–967 (2006).
Tepmongkol, S., Keelawat, S., Honsawek, S. & Ruangvejvorachai, P. Rosiglitazone effect on radioiodine uptake in thyroid carcinoma patients with high thyroglobulin but negative total body scan: a correlation with the expression of peroxisome proliferator-activated receptor-γ. Thyroid 18, 697–704 (2008).
Kroll, T. G. et al. PAX8-PPARγ1 fusion oncogene in human thyroid carcinoma. Science 289, 1357–1360 (2000).
Giordano, T. J. et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin. Cancer Res. 12, 1983–1993 (2006).
Lacroix, L. et al. Follicular thyroid tumors with the PAX8-PPARγ1 rearrangement display characteristic genetic alterations. Am. J. Pathol. 167, 223–231 (2005).
Erdmann, E., Charbonnel, B. & Wilcox, R. Thiazolidinediones and cardiovascular risk - a question of balance. Curr. Cardiol. Rev. 5, 155–165 (2009).
Grey, A. et al. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 92, 1305–1310 (2007).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006).
Schwartz, A. V. & Sellmeyer, D. E. Thiazolidinedione therapy gets complicated: is bone loss the price of improved insulin resistance? Diabetes Care 30, 1670–1671 (2007).
Schwartz, A. V. et al. Thiazolidinedione use and bone loss in older diabetic adults. J. Clin. Endocrinol. Metab. 91, 3349–3354 (2006).
Kodera, Y. et al. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J. Biol. Chem. 275, 33201–33204 (2000).
Zhang, Q., Zhou, H., Zhai, S. & Yan, B. Natural product-inspired synthesis of thiazolidine and thiazolidinone compounds and their anticancer activities. Curr. Pharm. Des. 16, 1826–1842 (2010).
Burton, J. D., Castillo, M. E., Goldenberg, D. M. & Blumenthal, R. D. Peroxisome proliferator-activated receptor-γ antagonists exhibit potent antiproliferative effects versus many hematopoietic and epithelial cancer cell lines. Anticancer Drugs 18, 525–534 (2007).
Lea, M. A., Sura, M. & Desbordes, C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) γ agonists and antagonists. Anticancer Res. 24, 2765–2771 (2004).
Schaefer, K. L. et al. Peroxisome proliferator-activated receptor γ inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Res. 65, 2251–2259 (2005).
Takahashi, H. et al. Inhibition of peroxisome proliferator-activated receptor γ activity in esophageal carcinoma cells results in a drastic decrease of invasive properties. Cancer Sci. 97, 854–860 (2006).
Burton, J. D., Goldenberg, D. M. & Blumenthal, R. D. Potential of peroxisome proliferator-activated receptor γ antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008, 494161 (2008).
Osborne, C. K. et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J. Natl Cancer Inst. 87, 746–750 (1995).
Kohno, H., Suzuki, R., Sugie, S. & Tanaka, T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer 5, 46 (2005).
Niho, N. et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 63, 6090–6095 (2003).
Tanaka, T. et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 61, 2424–2428 (2001).
Tenenbaum, A. et al. Does the lipid-lowering peroxisome proliferator-activated receptors ligand bezafibrate prevent colon cancer in patients with coronary artery disease? Cardiovasc. Diabetol. 7, 18 (2008). This study suggests that the pan-PPAR agonist bezafibrate may prevent colon cancer in humans, supporting the hypothesis that targeting all three PPARs may be suitable for chemoprevention.
Jackson, L. et al. Potential role for peroxisome proliferator activated receptor (PPAR) in preventing colon cancer. Gut 52, 1317–1322 (2003).
Khanim, F. L. et al. Combined bezafibrate and medroxyprogesterone acetate: potential novel therapy for acute myeloid leukaemia. PLoS ONE 4, e8147 (2009).
Hayden, R. E. et al. Treatment of primary CLL cells with bezafibrate and medroxyprogesterone acetate induces apoptosis and represses the pro-proliferative signal of CD40-ligand, in part through increased 15dΔ12,14, PGJ2 . Leukemia 23, 292–304 (2009).
Rubenstrunk, A., Hanf, R., Hum., D. W., Fruchart, J. C. & Staels, B. Safety issues and prospects for future generations of PPAR modulators. Biochim. Biophys. Acta 1771, 1065–1081 (2007).
Peraza, M. A., Burdick, A. D., Marin, H. E., Gonzalez, F. J. & Peters, J. M. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicol. Sci. 90, 269–295 (2006).
Still, K., Grabowski, P., Mackie, I., Perry, M. & Bishop, N. The peroxisome proliferator activator receptor α/δ agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif. Tissue Int. 83, 285–292 (2008).
Brautigam, K. et al. Combined treatment with TRAIL and PPARγ ligands overcomes chemoresistance of ovarian cancer cell lines. J. Cancer Res. Clin. Oncol. 137, 875–886 (2011).
Cesario, R. M., Stone, J., Yen, W. C., Bissonnette, R. P. & Lamph, W. W. Differentiation and growth inhibition mediated via the RXR:PPARγ heterodimer in colon cancer. Cancer Lett. 240, 225–233 (2006).
Crowe, D. L. & Chandraratna, R. A. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-α is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 6, R546–R555 (2004).
Desreumaux, P. et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor γ (PPARγ) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 193, 827–838 (2001).
Fu, H. et al. Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J. mice. Mol. Carcinog. 50, 913–921 (2011).
Girnun, G. D. et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell 11, 395–406 (2007).
Hamaguchi, N. et al. In vitro and in vivo therapeutic efficacy of the PPAR-γ agonist troglitazone in combination with cisplatin against malignant pleural mesothelioma cell growth. Cancer Sci. 101, 1955–1964 (2010).
Park, B. H., Lee, S. B., Stolz, D. B., Lee, Y. J. & Lee, B. C. Synergistic interactions between heregulin and peroxisome proliferator-activated receptor-γ (PPARγ) agonist in breast cancer cells. J. Biol. Chem. 286, 20087–20099 (2011).
Reddy, R. C. et al. Chemotherapeutic drugs induce PPARγ expression and show sequence-specific synergy with PPARγ ligands in inhibition of non-small cell lung cancer. Neoplasia 10, 597–603 (2008).
Tikoo, K., Kumar, P. & Gupta, J. Rosiglitazone synergizes anticancer activity of cisplatin and reduces its nephrotoxicity in 7, 12-dimethyl benz{a}anthracene (DMBA) induced breast cancer rats. BMC Cancer 9, 107 (2009).
Yamazaki, K. et al. Synergistic effects of RXR α and PPAR γ ligands to inhibit growth in human colon cancer cells--phosphorylated RXR α is a critical target for colon cancer management. Gut 56, 1557–1563 (2007).
Yokoyama, Y., Xin, B., Shigeto, T. & Mizunuma, H. Combination of ciglitazone, a peroxisome proliferator-activated receptor γ ligand, and cisplatin enhances the inhibition of growth of human ovarian cancers. J. Cancer Res. Clin. Oncol. 137, 1219–1228 (2011).
Fauconnet, S. et al. Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. J. Biol. Chem. 277, 23534–23543 (2002).
Meissner, M., Hrgovic, I., Doll, M. & Kaufmann, R. PPARδ agonists suppress angiogenesis in a VEGFR2-dependent manner. Arch. Dermatol. Res. 303, 41–47 (2011).
Abdollahi, A. et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc. Natl Acad. Sci. USA 104, 12890–12895 (2007).
Müller-Brüsselbach, S. et al. Deregulation of tumor angiogenesis and blockade of tumor growth in PPARβ-deficient mice. EMBO J. 26, 3686–3698 (2007).
Piqueras, L. et al. Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 27, 63–69 (2007).
Biscetti, F. et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)α and PPARγ induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes 57, 1394–1404 (2008).
Bishop-Bailey, D. & Hla, T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Δ12,14-prostaglandin J2 . J. Biol. Chem. 274, 17042–17048 (1999).
Chu, K. et al. Peroxisome proliferator-activated receptor-γ-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 1093, 208–218 (2006).
Huang, P. H. et al. Pioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic mice. Biomed. Pharmacother. 62, 46–52 (2008).
Xin, X., Yang, S., Kowalski, J. & Gerritsen, M. E. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 274, 9116–9121 (1999).
Chintalgattu, V., Harris, G. S., Akula, S. M. & Katwa, L. C. PPAR-γ agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc. Res. 74, 140–150 (2007).
Acknowledgements
The authors gratefully acknowledge J. Corell for technical assistance with the figures and P. Devchand for critical review and suggestions for the manuscript. The authors' research is supported by the US National Institutes of Health (CA124533, CA126826, CA141029, CA140369 and AA018863) to J.M.P., (R01CA148828) to Y.M.S. and the National Cancer Institute Intramural Research Program (ZIABC005561, ZIABC005562 and ZIABC005708) to F.J.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Peroxisome proliferator-activated receptors
-
(PPARs). This class of nuclear receptor acquired their name because the first receptor of this class identified (PPARα) mediates the phenomenon of the proliferation of peroxisomes observed in rodents given fibrates and other chemicals.
- Agonists
-
Compounds that bind to a receptor that invokes a biological response that is most often transcriptionally mediated. The specificity of an agonist is often defined by its ability to bind to the receptor at a given concentration and whether it is able to interact with a single receptor.
- Chemoprevention
-
The inhibition or prevention of disease by use of a drug or natural compound. Many chemopreventive agents show anti-inflammatory activities.
- Antagonists
-
Compounds that bind to a receptor and that block all known receptor activities induced by activation by an agonist. The potency of an antagonist is often defined by the concentration required to inhibit activation by an agonist.
Rights and permissions
About this article
Cite this article
Peters, J., Shah, Y. & Gonzalez, F. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer 12, 181–195 (2012). https://doi.org/10.1038/nrc3214
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3214
This article is cited by
-
Nuclear pore protein POM121 regulates subcellular localization and transcriptional activity of PPARγ
Cell Death & Disease (2024)
-
Enrichr in silico analysis of MS-based extracted candidate proteomic biomarkers highlights pathogenic pathways in systemic sclerosis
Scientific Reports (2023)
-
Bcl-2 inhibition combined with PPARα activation synergistically targets leukemic stem cell-like cells in acute myeloid leukemia
Cell Death & Disease (2023)
-
Targeting lipid-sensing nuclear receptors PPAR (α, γ, β/δ): HTVS and molecular docking/dynamics analysis of pharmacological ligands as potential pan-PPAR agonists
Molecular Diversity (2023)
-
Insight into the physiological and pathological roles of the aryl hydrocarbon receptor pathway in glucose homeostasis, insulin resistance, and diabetes development
Cellular & Molecular Biology Letters (2022)