Key Points
-
Oestrogen receptor (ER) subtypes (ERα and ERβ) influence the development and progression of hormone-related cancers by exerting distinct biological functions. ERα is associated with aberrant proliferation, inflammation and the development of malignancy. ERβ seems to oppose ERα actions on cell proliferation by modulating the expression of many ERα-regulated genes and exhibits antimigratory and anti-invasive properties in cancer cells.
-
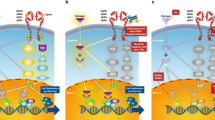
Multiple factors affect the ER-mediated regulation of gene expression and may account for the adverse and beneficial effects of oestrogens and anti-oestrogens. Both ER genomic and non-genomic actions often converge at certain regulatory sites of the adjacent ER-responsive genes. The final gene and the subsequent cancer biological responses may vary depending on the combination of transcription factors; the ratio and the cellular localization of ERα and ERβ; the expression levels of various co-regulators and signal transduction components; and the nature of extracellular stimuli. These variables are altered during cancer transformation and are divergent in different cancer cells.
-
Owing to the practical limitations in detection, only a few truncated ERα and ERβ variant isoforms have been examined in tumour samples and correlated with clinical outcome. Some of these variants are localized in the cytoplasm and plasma membrane, show variable expression in cancer tissues and influence cancer progression and response to therapy either through genomic pathways by modulating the activity of wild-type ERs or by interacting with the membrane and cytoplasmic signalling cascade.
-
Perturbation of ER subtype-specific expression has been detected in different stages of various types of cancer, with the levels of ERα and ERβ declining in most cancers as the disease develops. The hypermethylation of the ER promoters, microRNAs that target the ER mRNAs and increased proteasomal degradation are among the factors that are responsible for the reduced levels of ERs in cancer tissues.
-
ERα is the principal biomarker for the response of breast cancers to endocrine therapy, and its truncated isoform ERα-36 seems to confer resistance to tamoxifen. On-going research is trying to fully clarify the prognostic and predictive role of ERβ. So far, it seems that the nuclear wild-type ERβ complements ERα in predicting response to endocrine therapy and is associated with better overall outcome and the metastatic potential of breast and prostate cancer. The cytoplasmic ERβ2 (also known as ERβcx) isoform correlates with worse survival and metastatic phenotype.
-
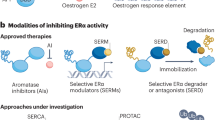
Insights into the mechanisms of ER action and regulation have suggested possible therapeutic approaches for hormone-related cancers. The development of selective ERα and ERβ agonists and antagonists, and alternative strategies that target the ER signalling beyond the ligand-binding activity, including as targets components of growth factor signalling, methylases, ubiquitin ligases, and chaperones are under investigation.
Abstract
By eliciting distinct transcriptional responses, the oestrogen receptors (ERs) ERα and ERβ exert opposite effects on cellular processes that include proliferation, apoptosis and migration and that differentially influence the development and the progression of cancer. Perturbation of ER subtype-specific expression has been detected in various types of cancer, and the differences in the expression of ERs are correlated with the clinical outcome. The changes in the bioavailability of ERs in tumours, together with their specific biological functions, promote the selective restoration of their activity as one of the major therapeutic approaches for hormone-dependent cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Heldring, N. et al. Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 87, 905–931 (2007). This review discusses new insights into the mechanism of ER action and the molecular mechanism of anti-oestrogen signalling.
Toft, D. & Gorski, J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc. Natl Acad. Sci. USA 55, 1574–1581 (1966).
Walter, P. et al. Cloning of the human estrogen receptor cDNA. Proc. Natl Acad. Sci. USA 82, 7889–7893 (1985).
Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J. A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA 93, 5925–5930 (1996).
Herynk, M. H. & Fuqua, S. A. Estrogen receptor mutations in human disease. Endocr. Rev. 25, 869–898 (2004). This reference extensively describes the splice variants and mutations of the human ER subtypes and illustrates their role in disease.
Bjornstrom, L. & Sjoberg, M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 19, 833–842 (2005).
Colditz, G. A. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J. Natl Cancer Inst. 90, 814–823 (1998).
Hankinson, S. E., Colditz, G. A. & Willett, W. C. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 6, 213–218 (2004).
Shang, Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nature Rev. Cancer 6, 360–368 (2006).
Ellem, S. J. & Risbridger, G. P. Treating prostate cancer: a rationale for targeting local oestrogens. Nature Rev. Cancer 7, 621–627 (2007). This article discusses the complexity of oestrogen action in the prostate and suggests the selective targeting of ER subtypes as a potential new and more effective therapeutic approach.
Fox, E. M., Davis, R. J. & Shupnik, M. A. ERβ in breast cancer—onlooker, passive player, or active protector? Steroids 73, 1039–1051 (2008).
Wong, N. A. et al. ERβ isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J. Pathol. 207, 53–60 (2005).
Musgrove, E. A. & Sutherland, R. L. Biological determinants of endocrine resistance in breast cancer. Nature Rev. Cancer 9, 631–643 (2009).
Speirs, V. et al. Clinical importance of estrogen receptor β isoforms in breast cancer. J. Clin. Oncol. 26, 5825–5826 (2008).
Stabile, L. P. et al. Combined analysis of estrogen receptor β-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin. Cancer Res. 17, 154–164 (2011). This article summarizes new clinical findings regarding the prognostic role of ERβ in lung cancer.
Leung, Y. K. et al. Estrogen receptor β2 and β5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr. Relat. Cancer 17, 675–689 (2010).
Britton, D. J. et al. Bidirectional cross talk between ERα and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res. Treat 96, 131–146 (2006).
Nonclercq, D., Journe, F., Body, J. J., Leclercq, G. & Laurent, G. Ligand-independent and agonist-mediated degradation of estrogen receptor-α in breast carcinoma cells: evidence for distinct degradative pathways. Mol. Cell Endocrinol. 227, 53–65 (2004).
Swedenborg, E., Power, K. A., Cai, W., Pongratz, I. & Ruegg, J. Regulation of estrogen receptor β activity and implications in health and disease. Cell. Mol. Life Sci. 66, 3873–3894 (2009).
Xu, J., Wu, R. C. & O'Malley, B. W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nature Rev. Cancer 9, 615–630 (2009).
Enmark, E. et al. Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 82, 4258–4265 (1997).
Gosden, J. R., Middleton, P. G. & Rout, D. Localization of the human oestrogen receptor gene to chromosome 6q24----q27 by in situ hybridization. Cytogenet. Cell Genet. 43, 218–220 (1986).
Biswas, D. K., Singh, S., Shi, Q., Pardee, A. B. & Iglehart, J. D. Crossroads of estrogen receptor and NF-κB signaling. Sci. STKE 2005, pe27 (2005).
Fox, E. M., Andrade, J. & Shupnik, M. A. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids 74, 622–627 (2009).
Carroll, J. S. et al. Genome-wide analysis of estrogen receptor binding sites. Nature Genet. 38, 1289–1297 (2006). This reference describes distinct mechanisms of ER-mediated gene regulation.
Menendez, D., Inga, A. & Resnick, M. A. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc. Natl Acad. Sci. USA 107, 1500–1505 (2010).
Sotgia, F. et al. Caveolin-1, mammary stem cells, and estrogen-dependent breast cancers. Cancer Res. 66, 10647–10651 (2006).
Chambliss, K. L. et al. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 87, e44–e52 (2000).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730 (2001).
Massarweh, S. & Schiff, R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin. Cancer Res. 13, 1950–1954 (2007). References 13 and 30 summarize and evaluate new insights into the mechanism of endocrine resistance based on global gene expression profiling approaches and functional genetic screens and suggest new therapeutic opportunities to target resistance and improve breast cancer disease outcomes.
Razandi, M., Pedram, A., Merchenthaler, I., Greene, G. L. & Levin, E. R. Plasma membrane estrogen receptors exist and functions as dimers. Mol. Endocrinol. 18, 2854–2865 (2004).
Kahlert, S. et al. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 275, 18447–18453 (2000).
Shupnik, M. A. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene 23, 7979–7989 (2004).
Song, R. X. et al. Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol. Endocrinol. 16, 116–127 (2002).
Smith, C. L., Nawaz, Z. & O'Malley, B. W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 11, 657–666 (1997).
Webb, P. et al. ERβ binds N-CoR in the presence of estrogens via an LXXLL-like motif in the N-CoR C-terminus. Nucl. Recept. 1, 4 (2003).
Bouras, T., Southey, M. C. & Venter, D. J. Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res. 61, 903–907 (2001).
Klinge, C. M., Jernigan, S. C., Mattingly, K. A., Risinger, K. E. & Zhang, J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J. Mol. Endocrinol. 33, 387–410 (2004).
Dubik, D. & Shiu, R. P. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene 7, 1587–1594 (1992).
Hartman, J. et al. Tumor repressive functions of estrogen receptor β in SW480 colon cancer cells. Cancer Res. 69, 6100–6106 (2009).
Liu, M. M. et al. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J. Biol. Chem. 277, 24353–24360 (2002).
Foley, E. F., Jazaeri, A. A., Shupnik, M. A., Jazaeri, O. & Rice, L. W. Selective loss of estrogen receptor β in malignant human colon. Cancer Res. 60, 245–248 (2000).
Zhu, X. et al. Dynamic regulation of estrogen receptor-β expression by DNA methylation during prostate cancer development and metastasis. Am. J. Pathol. 164, 2003–2012 (2004).
Rutherford, T. et al. Absence of estrogen receptor-β expression in metastatic ovarian cancer. Obstet. Gynecol. 96, 417–421 (2000).
Leav, I. et al. Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol. 159, 79–92 (2001).
Eeckhoute, J. et al. Positive cross-regulatory loop ties GATA-3 to estrogen receptor α expression in breast cancer. Cancer Res. 67, 6477–6483 (2007).
Guo, S. & Sonenshein, G. E. Forkhead box transcription factor FOXO3a regulates estrogen receptor α expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol. Cell Biol. 24, 8681–8690 (2004).
Madureira, P. A. et al. The Forkhead box M1 protein regulates the transcription of the estrogen receptor α in breast cancer cells. J. Biol. Chem. 281, 25167–25176 (2006).
Adams, B. D., Furneaux, H. & White, B. A. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERα) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol. Endocrinol. 21, 1132–1147 (2007).
Liu, W. H. et al. MicroRNA-18a prevents estrogen receptor-α expression, promoting proliferation of hepatocellular carcinoma cells. Gastroenterology 136, 683–693 (2009).
Zhao, J. J. et al. MicroRNA-221/222 negatively regulates estrogen receptor α and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 283, 31079–31086 (2008).
Al-Nakhle, H. et al. Estrogen receptor β1 expression is regulated by miR-92 in breast cancer. Cancer Res. 70, 4778–4784 (2010).
Pandey, D. P. & Picard, D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor α mRNA. Mol. Cell Biol. 29, 3783–3790 (2009). References 49–53 illustrate the role of miRNAs in the regulation of the expression of ER subtypes in cancer cells and tissues.
Richter, K. & Buchner, J. Hsp90: chaperoning signal transduction. J. Cell Physiol. 188, 281–290 (2001).
Duong, V. et al. Differential regulation of estrogen receptor α turnover and transactivation by Mdm2 and stress-inducing agents. Cancer Res. 67, 5513–5521 (2007).
Li, L., Li, Z., Howley, P. M. & Sacks, D. B. E6AP and calmodulin reciprocally regulate estrogen receptor stability. J. Biol. Chem. 281, 1978–1985 (2006).
Giamas, G. et al. Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nature Med. 17, 715–719 (2011).
Pan, X. et al. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nature Med. 17, 708–714 (2011). References 57 and 58 demonstrate how changes in ER protein stability alter cancer response to therapy.
Bocchinfuso, W. P. & Korach, K. S. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia 2, 323–334 (1997).
Korach, K. S. Insights from the study of animals lacking functional estrogen receptor. Science 266, 1524–1527 (1994).
Feng, Y., Manka, D., Wagner, K. U. & Khan, S. A. Estrogen receptor-α expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl Acad. Sci. USA 104, 14718–14723 (2007).
Bocchinfuso, W. P., Hively, W. P., Couse, J. F., Varmus, H. E. & Korach, K. S. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-α. Cancer Res. 59, 1869–1876 (1999).
Hewitt, S. C. et al. Lack of ductal development in the absence of functional estrogen receptor α delays mammary tumor formation induced by transgenic expression of ErbB2/neu. Cancer Res. 62, 2798–2805 (2002).
Miermont, A. M., Parrish, A. R. & Furth, P. A. Role of ERα in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis. Carcinogenesis 31, 1124–1131 (2010).
Yoshidome, K., Shibata, M. A., Couldrey, C., Korach, K. S. & Green, J. E. Estrogen promotes mammary tumor development in C3(1)/SV40 large T-antigen transgenic mice: paradoxical loss of estrogen receptorα expression during tumor progression. Cancer Res. 60, 6901–6910 (2000).
Risbridger, G. et al. Evidence that epithelial and mesenchymal estrogen receptor-α mediates effects of estrogen on prostatic epithelium. Dev. Biol. 229, 432–442 (2001).
Bianco, J. J., Handelsman, D. J., Pedersen, J. S. & Risbridger, G. P. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143, 4922–4933 (2002).
Bianco, J. J., McPherson, S. J., Wang, H., Prins, G. S. & Risbridger, G. P. Transient neonatal estrogen exposure to estrogen-deficient mice (aromatase knockout) reduces prostate weight and induces inflammation in late life. Am. J. Pathol. 168, 1869–1878 (2006).
Prins, G. S. et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res. 61, 6089–6097 (2001).
Gingrich, J. R. et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 56, 4096–4102 (1996).
Raghow, S., Hooshdaran, M. Z., Katiyar, S. & Steiner, M. S. Toremifene prevents prostate cancer in the transgenic adenocarcinoma of mouse prostate model. Cancer Res. 62, 1370–1376 (2002).
Castro-Rivera, E., Samudio, I. & Safe, S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 276, 30853–30861 (2001).
List, H. J. et al. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J. Biol. Chem. 276, 23763–23768 (2001).
Planas-Silva, M. D., Shang, Y., Donaher, J. L., Brown, M. & Weinberg, R. A. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 61, 3858–3862 (2001).
Levin, E. R. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol. 17, 309–317 (2003).
Driggers, P. H. & Segars, J. H. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol. Metab. 13, 422–427 (2002).
Forster, C. et al. Involvement of estrogen receptor β in terminal differentiation of mammary gland epithelium. Proc. Natl Acad. Sci. USA 99, 15578–15583 (2002).
Imamov, O., Lopatkin, N. A. & Gustafsson, J. A. Estrogen receptor β in prostate cancer. N. Engl. J. Med. 351, 2773–2774 (2004).
Imamov, O. et al. Estrogen receptorβ regulates epithelial cellular differentiation in the mouse ventral prostate. Proc. Natl Acad. Sci. USA 101, 9375–9380 (2004).
Weihua, Z. et al. A role for estrogen receptor β in the regulation of growth of the ventral prostate. Proc. Natl Acad. Sci. USA 98, 6330–6335 (2001).
Antal, M. C., Krust, A., Chambon, P. & Mark, M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc. Natl Acad. Sci. USA 105, 2433–2438 (2008).
Dupont, S. et al. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127, 4277–4291 (2000).
Nakajima, Y. et al. Estrogen regulates tumor growth through a nonclassical pathway that includes the transcription factors ERβ and KLF5. Sci. Signal. 4, ra22 (2011). This article demonstrates the tumour suppressive properties of ERβ and describes one of the mechanisms through which ERβ regulates tumour growth.
Strom, A. et al. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl Acad. Sci. USA 101, 1566–1571 (2004).
Lin, C. Y. et al. Inhibitory effects of estrogen receptor β on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 9, R25 (2007).
Lindberg, M. K. et al. Estrogen receptor (ER)-β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol. Endocrinol. 17, 203–208 (2003).
Williams, C., Edvardsson, K., Lewandowski, S. A., Strom, A. & Gustafsson, J. A. A genome-wide study of the repressive effects of estrogen receptor β on estrogen receptor α signaling in breast cancer cells. Oncogene 27, 1019–1032 (2008).
Hartman, J. et al. Estrogen receptor β inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 66, 11207–11213 (2006).
Thomas, C. G., Strom, A., Lindberg, K. & Gustafsson, J. A. Estrogen receptor β decreases survival of p53-defective cancer cells after DNA damage by impairing G2/M checkpoint signaling. Breast Cancer Res. Treat. 127, 417–427 (2011).
Hershberger, P. A. et al. Estrogen receptor β (ERβ) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J. Steroid Biochem. Mol. Biol. 116, 102–109 (2009).
Hou, Y. F. et al. ERβ exerts multiple stimulative effects on human breast carcinoma cells. Oncogene 23, 5799–5806 (2004).
Zhang, G. et al. Estrogen receptor β functions through nongenomic mechanisms in lung cancer cells. Mol. Endocrinol. 23, 146–156 (2009).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Barone, I., Brusco, L. & Fuqua, S. A. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin. Cancer Res. 16, 2702–2708 (2010).
Lin, S. L. et al. ER-α36, a variant of ER-α, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS ONE 5, e9013 (2010).
Poola, I. & Speirs, V. Expression of alternatively spliced estrogen receptor α mRNAs is increased in breast cancer tissues. J. Steroid Biochem. Mol. Biol. 78, 459–469 (2001).
Poola, I., Koduri, S., Chatra, S. & Clarke, R. Identification of twenty alternatively spliced estrogen receptor α mRNAs in breast cancer cell lines and tumors using splice targeted primer approach. J. Steroid Biochem. Mol. Biol. 72, 249–258 (2000).
Shi, L. et al. Expression of ER-α36, a novel variant of estrogen receptor α, and resistance to tamoxifen treatment in breast cancer. J. Clin. Oncol. 27, 3423–3429 (2009).
Castles, C. G., Fuqua, S. A., Klotz, D. M. & Hill, S. M. Expression of a constitutively active estrogen receptor variant in the estrogen receptor-negative BT-20 human breast cancer cell line. Cancer Res. 53, 5934–5939 (1993).
Desai, A. J. et al. Presence of exon 5-deleted oestrogen receptor in human breast cancer: functional analysis and clinical significance. Br. J. Cancer 75, 1173–1184 (1997).
Erenburg, I., Schachter, B., Mira y Lopez, R. & Ossowski, L. Loss of an estrogen receptor isoform (ER α δ 3) in breast cancer and the consequences of its reexpression: interference with estrogen-stimulated properties of malignant transformation. Mol. Endocrinol. 11, 2004–2015 (1997).
Moore, J. T. et al. Cloning and characterization of human estrogen receptor β isoforms. Biochem. Biophys. Res. Commun. 247, 75–78 (1998).
Omoto, Y., Eguchi, H., Yamamoto-Yamaguchi, Y. & Hayashi, S. Estrogen receptor (ER) β1 and ERβcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene 22, 5011–5020 (2003).
Peng, B., Lu, B., Leygue, E. & Murphy, L. C. Putative functional characteristics of human estrogen receptor-β isoforms. J. Mol. Endocrinol. 30, 13–29 (2003).
Green, C. A., Peter, M. B., Speirs, V. & Shaaban, A. M. The potential role of ER β isoforms in the clinical management of breast cancer. Histopathology 53, 374–380 (2008).
Shaaban, A. M. et al. Nuclear and cytoplasmic expression of ERβ1, ERβ2, and ERβ5 identifies distinct prognostic outcome for breast cancer patients. Clin. Cancer Res. 14, 5228–5235 (2008).
Saji, S. et al. Expression of estrogen receptor (ER) (β)cx protein in ER(α)-positive breast cancer: specific correlation with progesterone receptor. Cancer Res. 62, 4849–4853 (2002).
Yan, M., Rayoo, M., Takano, E. A. & Fox, S. B. Nuclear and cytoplasmic expressions of ERβ1 and ERβ2 are predictive of response to therapy and alters prognosis in familial breast cancers. Breast Cancer Res. Treat 126, 395–405 (2010).
Pettersson, K., Delaunay, F. & Gustafsson, J. A. Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene 19, 4970–4978 (2000).
Wang, X. et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nature Cell Biol. 9, 470–478 (2007).
Ye, Y. et al. ERα signaling through slug regulates E-cadherin and EMT. Oncogene 29, 1451–1462 (2010).
Mak, P. et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17, 319–332 (2010). An elegant proof of the anti-migratory and anti-invasive properties of ERβ, and a demonstration of its pivotal role in the early steps of the invasion and metastasis process.
Helguero, L. A. et al. Different roles of estrogen receptors α and β in the regulation of E-cadherin protein levels in a mouse mammary epithelial cell line. Cancer Res. 68, 8695–8704 (2008).
Lindberg, K. et al. Expression of estrogen receptor β increases integrin α1 and integrin β1 levels and enhances adhesion of breast cancer cells. J. Cell Physiol. 222, 156–167 (2010).
Shaaban, A. M. et al. Declining estrogen receptor-β expression defines malignant progression of human breast neoplasia. Am. J. Surg. Pathol. 27, 1502–1512 (2003).
Skliris, G. P. et al. Reduced expression of oestrogen receptor β in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J. Pathol. 201, 213–220 (2003).
Leung, Y. K., Mak, P., Hassan, S. & Ho, S. M. Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. Proc. Natl Acad. Sci. USA 103, 13162–13167 (2006).
Boyd, S. Remarks on oophorectomy in the treatment of cancer of the breast. Br. Med. J. 1, 257–262 (1899).
Bosland, M. C. et al. Chemoprevention strategies for prostate cancer. Eur. J. Cancer Prev. 11, S18–S27 (2002).
Couse, J. F. & Korach, K. S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417 (1999).
Deroo, B. J. & Korach, K. S. Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570 (2006).
Mah, V. et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 67, 10484–10490 (2007).
Henderson, B. E. & Feigelson, H. S. Hormonal carcinogenesis. Carcinogenesis 21, 427–433 (2000).
Jarred, R. A. et al. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol. Biomarkers Prev. 11, 1689–1696 (2002).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Johnston, S. R. & Dowsett, M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nature Rev. Cancer 3, 821–831 (2003).
Smith, M. R. et al. Selective aromatase inhibition for patients with androgen-independent prostate carcinoma. Cancer 95, 1864–1868 (2002).
Leygue, E., Dotzlaw, H., Watson, P. H. & Murphy, L. C. Altered estrogen receptor α and β messenger RNA expression during human breast tumorigenesis. Cancer Res. 58, 3197–3201 (1998).
Roger, P. et al. Dissociated overexpression of cathepsin D and estrogen receptor α in preinvasive mammary tumors. Hum. Pathol. 31, 593–600 (2000).
Chi, A., Chen, X., Chirala, M. & Younes, M. Differential expression of estrogen receptor β isoforms in human breast cancer tissue. Anticancer Res. 23, 211–216 (2003).
Esslimani-Sahla, M. et al. Increased estrogen receptor βcx expression during mammary carcinogenesis. Clin. Cancer Res. 11, 3170–3174 (2005).
Bonkhoff, H., Fixemer, T., Hunsicker, I. & Remberger, K. Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate 48, 285–291 (2001).
Hogdall, E. V. et al. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncol. Rep. 18, 1051–1059 (2007).
Issa, R. M. et al. Estrogen receptor gene amplification occurs rarely in ovarian cancer. Mod. Pathol. 22, 191–196 (2009).
Jazaeri, A. A. et al. Well-differentiated endometrial adenocarcinomas and poorly differentiated mixed mullerian tumors have altered ER and PR isoform expression. Oncogene 20, 6965–6969 (2001).
Saegusa, M. & Okayasu, I. Changes in expression of estrogen receptors α and β in relation to progesterone receptor and pS2 status in normal and malignant endometrium. Jpn. J. Cancer Res. 91, 510–518 (2000).
Tan, D. S., Lambros, M. B., Marchio, C. & Reis-Filho, J. S. ESR1 amplification in endometrial carcinomas: hope or hyperbole? J. Pathol. 216, 271–274 (2008).
Critchley, H. O. et al. Wild-type estrogen receptor (ERβ1) and the splice variant (ERβcx/β2) are both expressed within the human endometrium throughout the normal menstrual cycle. J. Clin. Endocrinol. Metab. 87, 5265–5273 (2002).
Skrzypczak, M. et al. Evaluation of mRNA expression of estrogen receptor β and its isoforms in human normal and neoplastic endometrium. Int. J. Cancer 110, 783–787 (2004).
Suzuki, F. et al. Loss of estrogen receptor β isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 99, 2365–2372 (2008).
Jassam, N., Bell, S. M., Speirs, V. & Quirke, P. Loss of expression of oestrogen receptor β in colon cancer and its association with Dukes' staging. Oncol. Rep. 14, 17–21 (2005).
Shah, Y. M. & Rowan, B. G. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (α) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol. Endocrinol. 19, 732–748 (2005).
Smith, C. L. & O'Malley, B. W. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71 (2004).
Picard, N. et al. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor β. Mol. Endocrinol. 22, 317–330 (2008).
Sauve, K., Lepage, J., Sanchez, M., Heveker, N. & Tremblay, A. Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res. 69, 5793–5800 (2009).
Skliris, G. P. et al. Immunohistochemical validation of multiple phospho-specific epitopes for estrogen receptor α (ERα) in tissue microarrays of ERα positive human breast carcinomas. Breast Cancer Res. Treat 118, 443–453 (2009).
Holm, C. et al. Phosphorylation of the oestrogen receptor α at serine 305 and prediction of tamoxifen resistance in breast cancer. J. Pathol. 217, 372–379 (2009).
Kok, M. et al. Estrogen receptor-α phosphorylation at serine-118 and tamoxifen response in breast cancer. J. Natl Cancer Inst. 101, 1725–1729 (2009).
Murphy, L. et al. Phospho-serine-118 estrogen receptor-α detection in human breast tumors in vivo. Clin. Cancer Res. 10, 1354–1359 (2004).
Murphy, L. C., Niu, Y., Snell, L. & Watson, P. Phospho-serine-118 estrogen receptor-α expression is associated with better disease outcome in women treated with tamoxifen. Clin. Cancer Res. 10, 5902–5906 (2004).
Sarwar, N. et al. Phosphorylation of ERα at serine 118 in primary breast cancer and in tamoxifen-resistant tumours is indicative of a complex role for ERα phosphorylation in breast cancer progression. Endocr. Relat. Cancer 13, 851–861 (2006).
Yamashita, H. et al. Phosphorylation of estrogen receptor α serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast Cancer Res. 7, R753–R764 (2005).
Yamashita, H. et al. Low phosphorylation of estrogen receptor α (ERα) serine 118 and high phosphorylation of ERα serine 167 improve survival in ER-positive breast cancer. Endocr. Relat. Cancer 15, 755–763 (2008).
Hamilton-Burke, W. et al. Phosphorylation of estrogen receptor β at serine 105 is associated with good prognosis in breast cancer. Am. J. Pathol. 177, 1079–1086 (2010). References 147–155 provide clinical evidence that the phosphorylation status of ER subtypes can be used in prognosis of breast cancer.
Platet, N., Cathiard, A. M., Gleizes, M. & Garcia, M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit. Rev. Oncol. Hematol. 51, 55–67 (2004).
Butt, A. J., McNeil, C. M., Musgrove, E. A. & Sutherland, R. L. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr. Relat. Cancer 12, S47–S59 (2005).
Musgrove, E. A. et al. Identification of functional networks of estrogen- and c-Myc-responsive genes and their relationship to response to tamoxifen therapy in breast cancer. PLoS ONE 3, e2987 (2008).
Nehra, R. et al. BCL2 and CASP8 regulation by NF-κB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells. FASEB J. 24, 2040–2055 (2010).
Hurtado, A. et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456, U663–U693 (2008). This reference indicates the crucial role of co-regulatory proteins in determining ER-mediated transcriptional responses.
Ali, S. & Coombes, R. C. Endocrine-responsive breast cancer and strategies for combating resistance. Nature Rev. Cancer 2, 101–112 (2002).
Osborne, C. K. et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl Cancer Inst. 95, 353–361 (2003).
Ring, A. & Dowsett, M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer 11, 643–658 (2004).
Ishii, Y. et al. Bortezomib enhances the efficacy of fulvestrant by amplifying the aggregation of the estrogen receptor, which leads to a pro-apoptotic unfolded protein response. Clin. Cancer Res. 17, 2292 (2011).
Scriven, P. et al. Activation and clinical significance of the unfolded protein response in breast cancer. Br. J. Cancer 101, 1692–1698 (2009).
Honma, N. et al. Clinical importance of estrogen receptor-β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 26, 3727–3734 (2008).
Novelli, F. et al. A divergent role for estrogen receptor-β in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 10, R74 (2008). References 106, 166 and 167 represent three recent studies which, by analysing large number of samples using well-validated antibodies, provide clinical evidence for the prognostic and predictive role of ERβ in breast cancer.
Horvath, G., Leser, G., Hahlin, M. & Henriksson, M. Exon deletions and variants of human estrogen receptor mRNA in endometrial hyperplasia and adenocarcinoma. Int. J. Gynecol. Cancer 10, 128–136 (2000).
Kawai, H. et al. Estrogen receptor α and β are prognostic factors in non-small cell lung cancer. Clin. Cancer Res. 11, 5084–5089 (2005).
Nose, N. et al. Association between estrogen receptor-β expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J. Clin. Oncol. 27, 411–417 (2009).
Nose, N., Uramoto, H., Iwata, T., Hanagiri, T. & Yasumoto, K. Expression of estrogen receptor β predicts a clinical response and longer progression-free survival after treatment with EGFR-TKI for adenocarcinoma of the lung. Lung Cancer 71, 350–355 (2010).
Raso, M. G. et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin. Cancer Res. 15, 5359–5368 (2009).
Schwartz, A. G. et al. Nuclear estrogen receptor β in lung cancer: expression and survival differences by sex. Clin. Cancer Res. 11, 7280–7287 (2005).
Skov, B. G., Fischer, B. M. & Pappot, H. Oestrogen receptor β over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 59, 88–94 (2008).
Wu, C. T., Chang, Y. L., Shih, J. Y. & Lee, Y. C. The significance of estrogen receptor β in 301 surgically treated non-small cell lung cancers. J. Thorac. Cardiovasc. Surg. 130, 979–986 (2005).
Alonso, L. et al. Gonadotropin and steroid receptors as prognostic factors in advanced ovarian cancer: a retrospective study. Clin. Transl. Oncol. 11, 748–752 (2009).
Darb-Esfahani, S. et al. Estrogen receptor 1 mRNA is a prognostic factor in ovarian carcinoma: determination by kinetic PCR in formalin-fixed paraffin-embedded tissue. Endocr. Relat. Cancer 16, 1229–1239 (2009).
Faggad, A. et al. Prognostic significance of Dicer expression in ovarian cancer-link to global microRNA changes and oestrogen receptor expression. J. Pathol. 220, 382–391 (2010).
Zamagni, C. et al. Oestrogen receptor 1 mRNA is a prognostic factor in ovarian cancer patients treated with neo-adjuvant chemotherapy: determination by array and kinetic PCR in fresh tissue biopsies. Endocr. Relat. Cancer 16, 1241–1249 (2009).
Burges, A. et al. Prognostic significance of estrogen receptor α and β expression in human serous carcinomas of the ovary. Arch. Gynecol. Obstet. 281, 511–517 (2010).
Shabani, N. et al. Prognostic significance of oestrogen receptor α (ERα) and β (ERβ), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur. J. Cancer 43, 2434–2444 (2007).
Suthipintawong, C., Wejaranayang, C. & Vipupinyo, C. Prognostic significance of ER, PR, Ki67, c-erbB-2, and p53 in endometrial carcinoma. J. Med. Assoc. Thai 91, 1779–1784 (2008).
Barone, M. et al. Dietary-induced ERβ upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis 31, 269–274 (2010).
Giroux, V., Lemay, F., Bernatchez, G., Robitaille, Y. & Carrier, J. C. Estrogen receptor β deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int. J. Cancer 123, 303–311 (2008).
Martineti, V. et al. ERβ is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr. Relat. Cancer 12, 455–469 (2005).
Wilkins, H. R., Doucet, K., Duke, V., Morra, A. & Johnson, N. Estrogen prevents sustained COLO-205 human colon cancer cell growth by inducing apoptosis, decreasing c-myb protein, and decreasing transcription of the anti-apoptotic protein bcl-2. Tumour Biol. 31, 16–22 (2010).
Bardin, A. et al. Involvement of estrogen receptor β in ovarian carcinogenesis. Cancer Res. 64, 5861–5869 (2004).
Fan, X. et al. Gonadotropin-positive pituitary tumors accompanied by ovarian tumors in aging female ERβ−/− mice. Proc. Natl Acad. Sci. USA 107, 6453–6458 (2010).
Acknowledgements
Research in the authors' laboratory is supported by the Welch Foundation and the Texas Emergency Technology Fund, under Agreement No 300-9-1958.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.-A.G. is a consultant with Karo Bio AB and BioNovo. C.T. declares no competing financial interests.
Related links
Glossary
- Co-activators
-
Proteins that increase gene expression by binding to a transcription factor that binds DNA through its DNA-binding domain.
- Co-repressors
-
Proteins that decrease gene expression by binding to a transcription factor that contains a DNA-binding domain.
- 'Pure' anti-oestrogens
-
Drugs that bind the oestrogen receptor, thereby blocking the effect of oestrogen, but that have no detectable oestrogen-like effects. Most have a steroidal structure.
- Gleason grade
-
The assignment of a number between 1 and 5 to indicate the degree of differentiation of the cells in the cancer specimen. It is used to establish the Gleason score. Cancers with a higher Gleason score are more aggressive and have a worse prognosis.
- Hormone replacement therapy
-
(HRT). The administration of hormones to correct a deficiency, such as postmenopausal oestrogen replacement therapy.
- SERMs
-
Drugs that bind the oestrogen receptor and thereby block the effects of oestrogen on tissues such as the breast but that function similarly to oestrogen in other tissues, such as the endometrium. These drugs are not steroidal in structure.
- Aromatase inhibitors
-
Drugs that block aromatase, the enzyme that converts androgens to oestrogens in tissues including the breast and adipose tissue.
- Unfolded protein response
-
A cellular response to stress that senses misfolded proteins in the endoplasmic reticulum. It activates pathways that help cells to survive the toxicity that is caused by unfolded proteins or to activate mechanisms of cell death.
Rights and permissions
About this article
Cite this article
Thomas, C., Gustafsson, JÅ. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 11, 597–608 (2011). https://doi.org/10.1038/nrc3093
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3093
This article is cited by
-
The increased risk of colorectal cancer in the women who underwent hysterectomy from the South Korean National Health Insurance Database
BMC Women's Health (2023)
-
EYA4 promotes breast cancer progression and metastasis through its role in replication stress avoidance
Molecular Cancer (2023)
-
Deep learning-based system for automatic prediction of triple-negative breast cancer from ultrasound images
Medical & Biological Engineering & Computing (2023)
-
Landscape of NcRNAs involved in drug resistance of breast cancer
Clinical and Translational Oncology (2023)
-
Determination of estrogen receptor alpha gene (ESR1) polymorphism and its relation to systemic lupus erythematosus disease status
Egyptian Rheumatology and Rehabilitation (2022)