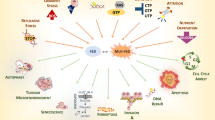
Key Points
-
p53 is an evolutionary ancient transcription factor, the primordial function of which in early metazoans may have been to coordinate transcriptional responses to stress and damage.
-
Vertebrate p53 is activated by many types of stress and damage. In its basal 'unactivated' state it also controls various normal physiological functions.
-
In vertebrates, p53 acts as an important tumour suppressor and either it or its attendant upstream or downstream pathways are functionally inactivated in virtually all cancers.
-
The extent to which the roles of p53 in tumour suppression, stress or damage responses and normal physiology are interdependent or overlap mechanistically is unclear.
-
Vertebrate p53 is highly pleiotropic and presides over a diverse range of contingent cell responses. Some of these responses are ostensibly antithetical — for example, p53 coordinates both the 'pause–repair–recovery' and 'senescence and/or cell death' responses to genotoxic injury.
-
Such multifunctionality arises from the tortuous evolutionary legacy of p53, and may have led to compromises that degrade the efficacy of p53 as a tumour suppressor.
-
The effectiveness of p53-mediated tumour suppression in vertebrates relies on the consistent and reliable activation of the p53 pathway by oncogenic signalling but never by normal mitogenic signals.
-
The ARF tumour suppressor seems to be the pre-eminent mediator of p53 activation in cancer cells. ARF has evolved to be specifically induced only by oncogenic signals, which are persistent and obligate attributes of cancer cells throughout their genesis and subsequent evolution.
-
However, the trigger for ARF (and hence, the p53 pathway) in tumour cells is not the abnormal persistence of growth signals, which is what makes signals oncogenic, but the aberrantly high signal strength, which is a frequent — but not unfailing — correlate of oncogenesis.
-
Therefore, the slapdash evolution of p53-mediated tumour suppression has incorporated a fundamental flaw — it senses only a symptom of oncogenic signalling rather than the oncogenic signal itself.
Abstract
Cancers are rare because their evolution is actively restrained by a range of tumour suppressors. Of these p53 seems unusually crucial as either it or its attendant upstream or downstream pathways are inactivated in virtually all cancers. p53 is an evolutionarily ancient coordinator of metazoan stress responses. Its role in tumour suppression is likely to be a relatively recent adaptation, which is only necessary when large, long-lived organisms acquired the sufficient size and somatic regenerative capacity to necessitate specific mechanisms to reign in rogue proliferating cells. However, such evolutionary reappropriation of this venerable transcription factor entails compromises that restrict its efficacy as a tumour suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nedelcu, A. M. & Tan, C. Early diversification and complex evolutionary history of the p53 tumor suppressor gene family. Dev. Genes. Evol. 217, 801–806 (2007).
Fernandes, A. D. & Atchley, W. R. Biochemical and functional evidence of p53 homology is inconsistent with molecular phylogenetics for distant sequences. J. Mol. Evol. 67, 51–67 (2008).
Hu, W., Feng, Z., Teresky, A. K. & Levine, A. J. p53 regulates maternal reproduction through LIF. Nature 450, 721–724 (2007).
Kanfi, Y. et al. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett. 582, 2417–2423 (2008).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Tasdemir, E. et al. Regulation of autophagy by cytoplasmic p53. Nature Cell. Biol. 10, 676–687 (2008).
Godar, S. et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell 134, 62–73 (2008).
Gatza, C., Moore, L., Dumble, M. & Donehower, L. Tumor suppressor dosage regulates stem cell dynamics during aging. Cell Cycle 6, 52–55 (2007).
Danilova, N., Sakamoto, K. M. & Lin, S. p53 family in development. Mech. Dev. 125, 919–931 (2008).
Nordstrom, W. & Abrams, J. M. Guardian ancestry: fly p53 and damage-inducible apoptosis. Cell Death Differ. 7, 1035–1038 (2000).
Brodsky, M. H., Sekelsky, J. J., Tsang, G., Hawley, R. S. & Rubin, G. M. mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14, 666–678 (2000).
Borel, F., Lohez, O. D., Lacroix, F. B. & Margolis, R. L. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc. Natl Acad. Sci. USA 99, 9819–9824 (2002).
Donehower, L. A. et al. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 9, 882–895 (1995).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005).
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005).
Lavin, M. F. & Gueven, N. The complexity of p53 stabilization and activation. Cell Death Differ. 13, 941–950 (2006).
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006). This study presents the first in vivo evidence that the p53-mediated DNA damage response may be dispensable for p53-mediated tumour suppression.
Marino, S., Vooijs, M., van Der Gulden, H., Jonkers, J. & Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000).
Hinkal, G., Parikh, N. & Donehower, L. A. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS ONE 4, e6654 (2009).
Quelle, D. E., Zindy, F., Ashmun, R. A. & Sherr, C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Kamijo, T. et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl Acad. Sci. USA 95, 8292–8297 (1998).
Pomerantz, J. et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92, 713–723 (1998).
Stott, F. J. et al. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17, 5001–5014 (1998).
Zhang, Y., Xiong, Y. & Yarbrough, W. G. ARF promotes MDM2 degradation and stabilizes p53: ARF–INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92, 725–734 (1998).
Zindy, F. et al. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc. Natl Acad. Sci. USA 100, 15930–15935 (2003).
Efeyan, A., Garcia-Cao, I., Herranz, D., Velasco-Miguel, S. & Serrano, M. Tumour biology: policing of oncogene activity by p53. Nature 443, 159 (2006).
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Kamijo, T., Bodner, S., van de Kamp, E., Randle, D. & Sherr, C. Tumor spectrum in ARF-deficient mice. Cancer Res. 59, 2217–2222 (1999).
Martins, C. P., Brown-Swigart, L. & Evan, G. I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323–1334 (2006).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). References 31–33 offered the first in vivo evidence for the potential therapeutic benefit of restoring p53 function in established cancers.
Garcia-Cao, I. et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21, 6225–6235 (2002). A groundbreaking study exploring the possibility of increasing p53-mediated tumour suppression by recombineering through the provision of additional germline copies of Trp53.
Brookes, S. et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21, 2936–2945 (2002).
Wei, W., Hemmer, R. M. & Sedivy, J. M. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21, 6748–6757 (2001).
DePinho, R. A. The age of cancer. Nature 408, 248–254 (2000).
Prowse, K. R. & Greider, C. W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl Acad. Sci. USA 92, 4818–4822 (1995).
Gromley, A., Churchman, M. L., Zindy, F. & Sherr, C. J. Transient expression of the Arf tumor suppressor during male germ cell and eye development in Arf–Cre reporter mice. Proc. Natl Acad. Sci. USA 106, 6285–6290 (2009).
McKeller, R. N. et al. The Arf tumor suppressor gene promotes hyaloid vascular regression during mouse eye development. Proc. Natl Acad. Sci. USA 99, 3848–3853 (2002).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397, 164–168 (1999).
Jacobs, J. J. et al. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 13, 2678–2690 (1999).
Gil, J., Bernard, D., Martinez, D. & Beach, D. Polycomb CBX7 has a unifying role in cellular lifespan. Nature Cell Biol. 6, 67–72 (2004).
Jacobs, J. J. et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19 ARF) and is amplified in a subset of human breast cancers. Nature Genet. 26, 291–299 (2000).
Aslanian, A., Iaquinta, P. J., Verona, R. & Lees, J. A. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18, 1413–1422 (2004).
Maeda, T. et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature 433, 278–285 (2005).
Iaquinta, P. J., Aslanian, A. & Lees, J. A. Regulation of the Arf/p53 tumor surveillance network by E2F. Cold Spring Harb. Symp. Quant. Biol. 70, 309–316 (2005).
Sreeramaneni, R., Chaudhry, A., McMahon, M., Sherr, C. J. & Inoue, K. Ras–Raf–Arf signaling critically depends on the Dmp1 transcription factor. Mol. Cell. Biol. 25, 220–232 (2005).
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002).
Lawlor, E. R. et al. Reversible kinetic analysis of Myc targets in vivo provides novel insights into Myc-mediated tumorigenesis. Cancer Res. 66, 4591–4601 (2006).
Murphy, D. J. et al. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 14, 447–457 (2008). By showing that low-level, deregulated Myc has pervasive tumorigenic activity but fails to engage apoptosis or ARF–p53 in vivo , this study illustrated how signal intensity is the crucial factor that determines effective engagement of tumour suppression.
Sarkisian, C. J. et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nature Cell Biol. 9, 493–505 (2007). This study exposed the paradox that high-level expression of oncogenic Ras confers a potent selective disadvantage on cells because it triggers the ARF–p53 tumour suppressor response, whereas low levels of oncogenic Ras do not.
Pietsch, E. C., Sykes, S. M., McMahon, S. B. & Murphy, M. E. The p53 family and programmed cell death. Oncogene 27, 6507–6521 (2008).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nature Rev. Cancer 2, 594–604 (2002).
Finch, A. et al. Bcl-XL gain of function and p19ARF loss of function cooperate oncogenically with Myc in vivo by distinct mechanisms. Cancer Cell 10, 113–120 (2006).
Song, H., Hollstein, M. & Xu, Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nature Cell Biol. 9, 573–580 (2007).
Strano, S. et al. Mutant p53: an oncogenic transcription factor. Oncogene 26, 2212–2219 (2007).
Xu, Y. Induction of genetic instability by gain-of-function p53 cancer mutants. Oncogene 27, 3501–3507 (2008).
Lozano, G. The oncogenic roles of p53 mutants in mouse models. Curr. Opin. Genet. Dev. 17, 66–70 (2007).
Humbey, O. et al. The ARF tumor suppressor can promote the progression of some tumors. Cancer Res. 68, 9608–9613 (2008).
LaBaer, J. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
Ganguli, G. & Wasylyk, B. p53-independent functions of MDM2. Mol. Cancer Res. 1, 1027–1035 (2003).
Szymanska, K. & Hainaut, P. TP53 and mutations in human cancer. Acta Biochim. Pol. 50, 231–238 (2003).
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Matheu, A. et al. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Dev. 18, 2736–2746 (2004).
Munoz-Fontela, C. et al. Resistance to viral infection of super p53 mice. Oncogene 24, 3059–3062 (2005).
Matheu, A. et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 (2007).
Tyner, S. D. et al., p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002). The fascinating, if controversial, study that first raised the possibility that increasing overall p53 activity might increase tumour suppression, albeit at the expense of accelerated aging.
Courtois, S. et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 21, 6722–6728 (2002).
Yin, Y., Stephen, C. W., Luciani, M. G. & Fahraeus, R. p53 stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nature Cell Biol. 4, 462–467 (2002).
Maier, B. et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306–319 (2004).
Medrano, S., Burns-Cusato, M., Atienza, M. B., Rahimi, D. & Scrable, H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol. Aging 30, 483–497 (2009).
Campisi, J. Cancer and ageing: rival demons? Nature Rev. Cancer 3, 339–349 (2003).
Williams, G. Pleiotropy, natural selection and the evolution of senescence. Evolution 11, 398–411 (1957).
Gentry, A. & Venkatachalam, S. Complicating the role of p53 in aging. Aging Cell 4, 157–160 (2005).
Vijg, J. & Hasty, P. Aging and p53: getting it straight. A commentary on a recent paper by Gentry and Venkatachalam. Aging Cell 4, 331–333 (2005).
Mendrysa, S. M. et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 20, 16–21 (2006). In this study, the authors use a hypomorphic allele of Mdm2 to augment intrinsic p53 activity, showing increased tumour suppression without a concomitant deficit in longevity.
Alt, J. R., Greiner, T. C., Cleveland, J. L. & Eischen, C. M. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 22, 1442–1450 (2003).
Terzian, T. et al., Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol. Cell. Biol. 27, 5479–5485 (2007).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005).
Jones, S., Roe, A., Donehower, L. & Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206–208 (1995).
Montes de Oca Luna, R., Wagner, D. & Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206 (1995).
Parant, J. et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nature Genet. 29, 92–95 (2001).
Boesten, L. S. et al. Mdm2, but not Mdm4, protects terminally differentiated smooth muscle cells from p53-mediated caspase-3-independent cell death. Cell Death Differ. 13, 2089–2098 (2006).
Francoz, S. et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc. Natl Acad. Sci. USA 103, 3232–3237 (2006).
Xiong, S., Van Pelt, C. S., Elizondo-Fraire, A. C., Liu, G. & Lozano, G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc. Natl Acad. Sci. USA 103, 3226–3231 (2006).
Ringshausen, I., Oshea, C., Finch, A., Swigart, L. & Evan, G. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 10, 501–514 (2006).
Suh, E. et al. p63 protects the female germ line during meiotic arrest. Nature 444, 624–628 (2006).
Morris, S. C. The fossil record and the early evolution of the Metazoa. Nature 361, 219–225 (1993).
Acknowledgements
The authors thank the Ellison Medical Foundation for support. Melissa R. Junttila is the Enrique Cepero, Ph.D. Fellow of the Damon Runyon Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
NCI Nature Protein Interaction Database (PID)
FURTHER INFORMATION
International Agency for Research on Cancer (IARC) TP53 database
Glossary
- Cnidarians–bilaterians
-
Cinidarians comprise an animal phylum of ∼9,000 radially symmetrical, mostly marine organisms. Most other animals are bilaterally symmetrical and are classed as bilateria. The cnidarians and bilaterians last shared a common ancestor ∼570–700 million years ago.
- Squelching
-
Interference of one transcriptional activator by another is called squelching, and is caused by competition for binding a scarce factor.
Rights and permissions
About this article
Cite this article
Junttila, M., Evan, G. p53 — a Jack of all trades but master of none. Nat Rev Cancer 9, 821–829 (2009). https://doi.org/10.1038/nrc2728
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2728
This article is cited by
-
Convergent TP53 loss and evolvability in cancer
BMC Ecology and Evolution (2023)
-
Evolutionary determinants of curability in cancer
Nature Ecology & Evolution (2023)
-
A novel survival model based on a Ferroptosis-related gene signature for predicting overall survival in bladder cancer
BMC Cancer (2021)
-
Crosstalk between Plk1, p53, cell cycle, and G2/M DNA damage checkpoint regulation in cancer: computational modeling and analysis
npj Systems Biology and Applications (2021)
-
miR-539 activates the SAPK/JNK signaling pathway to promote ferropotosis in colorectal cancer by directly targeting TIPE
Cell Death Discovery (2021)