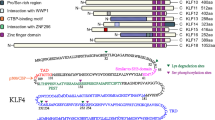
Key Points
-
Krüppel-like transcription factors (KLFs) regulate the expression of many genes, including those that are involved in differentiation and cell-cycle arrest.
-
One KLF family member, KLF4, possesses tumour-suppressor-like properties, as the gene that encodes it is deleted or methylated (silenced) in human gastrointestinal tract tumours. In mice, deletion of Klf4 in the gastric compartment induces hyperplasia and polyps.
-
Ectopic expression of KLF4 induces cell-cycle arrest in a p21-dependent manner.
-
KLF4 also seems to function as a dominant oncogene, as it is often overexpressed in human breast tumours and squamous cell carcinomas, and can also contribute to oncogenic transformation of cultured cells.
-
Loss of p21 is sufficient to convert KLF4 from an inhibitor of proliferation into a transforming oncogene in vitro.
-
p21 might represent a nodal point for signals from multiple factors with opposing, context-dependent functions in cancer, including transforming growth factor-β, Notch, Runx and Ras.
-
Although CDKN1A (cyclin-dependent kinase inhibitor 1A, the gene that encodes p21) is a target gene of many key tumour-suppressor pathways, it is not often lost or mutated in human tumours.
-
p21 itself also possesses opposing functions that might both counteract and contribute to cancer progression.
-
Partial, but not complete, loss of p21 might provide a selective advantage to tumour cells, which could be related to the proposed roles for p21 in suppressing apoptosis and inducing cell-cycle arrest.
Abstract
Krüppel-like factors are transcriptional regulators that influence several cellular functions, including proliferation. Recent studies have shown that one family member, KLF4, can function both as a tumour suppressor and an oncogene. The ability of KLF4 to affect the levels of expression of the cell-cycle regulator p21 seems to be involved, in that this protein might function as a switch that determines the outcome of KLF4 signalling. Is this role of p21 restricted to KLF4, or does p21 represent a nodal point for signals from multiple other factors with opposing functions in cancer?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Siegel, P. M. & Massague, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nature Rev. Cancer 3, 807–820 (2003).
Radtke, F. & Raj, K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nature Rev. Cancer 3, 756–767 (2003).
Blyth, K., Cameron, E. R. & Neil, J. C. The RUNX genes: gain or loss of function in cancer. Nature Rev. Cancer 5, 376–387 (2005).
DeGregori, J. The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochim. Biophys. Acta 1602, 131–150 (2002).
Roninson, I. B. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 179, 1–14 (2002).
Gloor, H. Schadigungsmuster eines Lethalfaktors (Kr) von Drosophila melanogaster. Arch. Jul. Klaus Stiftung 25, 38–44 (1950).
Turner, J. & Crossley, M. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem. Sci. 24, 236–240 (1999).
Philipsen, S. & Suske, G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 27, 2991–3000 (1999).
Dang, D. T., Pevsner, J. & Yang, V. W. The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 32, 1103–1121 (2000).
Black, A. R., Black, J. D. & Azizkhan-Clifford, J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell. Physiol. 188, 143–160 (2001).
Kaczynski, J., Cook, T. & Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 4, 206 (2003).
Suske, G., Bruford, E. & Philipsen, S. Mammalian SP/KLF transcription factors: bring in the family. Genomics 85, 551–556 (2005).
Narla, G. et al. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science 294, 2563–2566 (2001). Identifies KLF6 as a potential tumour suppressor.
Ghaleb, A. M. et al. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell. Res. 15, 92–96 (2005).
Wu, J. & Lingrel, J. B. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene 23, 8088–8096 (2004).
Wang, F. et al. Transcriptional repression of WEE1 by Kruppel-like factor 2 is involved in DNA damage-induced apoptosis. Oncogene 24, 3875–3885 (2005).
Bateman, N. W., Tan, D., Pestell, R. G., Black, J. D. & Black, A. R. Intestinal tumor progression is associated with altered function of KLF5. J. Biol. Chem. 279, 12093–12101 (2004).
Laub, F. et al. Developmental expression of mouse Kruppel-like transcription factor KLF7 suggests a potential role in neurogenesis. Dev. Biol. 233, 305–318 (2001).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The p21 CDK-interacting protein cip1 is a potent inhibitor of g1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Gu, Y., Turck, C. W. & Morgan, D. O. Inhibition of CDK2 activity in vivo by an associated 20k regulatory subunit. Nature 366, 707–710 (1993).
Noda, A., Ning, Y., Venable, S. F., Pereira-Smith, O. M. & Smith, J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell. Res. 211, 90–98 (1994).
Zhang, W. et al. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J. Biol. Chem. 275, 18391–18398 (2000).
Chen, X. et al. Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J. Biol. Chem. 276, 30423–30428 (2001).
Shiohara, M. et al. Absence of WAF1 mutations in a variety of human malignancies. Blood 84, 3781–3784 (1994).
Shields, J. M., Christy, R. J. & Yang, V. W. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J. Biol. Chem. 271, 20009–20017 (1996).
Garrett-Sinha, L. A., Eberspaecher, H., Seldin, M. F. & de Crombrugghe, B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J. Biol. Chem. 271, 31384–31390 (1996).
Chen, X., Whitney, E. M., Gao, S. Y. & Yang, V. W. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J. Mol. Biol. 326, 665–677 (2003).
Segre, J. A., Bauer, C. & Fuchs, E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nature Genet. 22, 356–360 (1999).
Katz, J. P. et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129, 2619–2628 (2002).
Ton-That, H., Kaestner, K. H., Shields, J. M., Mahatanankoon, C. S. & Yang, V. W. Expression of the gut-enriched Kruppel-like factor gene during development and intestinal tumorigenesis. FEBS Lett. 419, 239–243 (1997).
Dang, D. T. et al. Decreased expression of the gut-enriched Kruppel-like factor gene in intestinal adenomas of multiple intestinal neoplasia mice and in colonic adenomas of familial adenomatous polyposis patients. FEBS Lett. 476, 203–207 (2000).
Ohnishi, S. et al. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem. Biophys. Res. Commun. 308, 251–256 (2003).
Shie, J. L. et al. Role of gut-enriched Kruppel-like factor in colonic cell growth and differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G806–G814 (2000).
Zhao, W. et al. Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene 23, 395–402 (2004).
Wei, D. et al. Drastic down-regulation of Kruppel-like factor 4 expression Is critical in human gastric cancer development and progression. Cancer Res. 65, 2746–2754 (2005). References 35 and 36 show that KLF4 is deleted in gastrointestinal cancers.
Katz, J. P. et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology 128, 935–945 (2005). Shows that KLF4-deficiency results in gastric polyps in mice.
Dang, D. T., Mahatan, C. S., Dang, L. H., Agboola, I. A. & Yang, V. W. Expression of the gut-enriched Kruppel-like factor (Kruppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene 20, 4884–4890 (2001).
Xiong, Y. et al. p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704 (1993).
Rowland, B. D., Bernards, R. & Peeper, D. S. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nature Cell Biol. 7, 1074–1082 (2005). Identifies the mechanism by which KLF4 switches from a growth inhibitor to a transforming oncogene in vitro.
Okano, J. et al. The Kruppel-like transcriptional factors Zf9 and GKLF coactivate the human keratin 4 promoter and physically interact. FEBS Lett. 473, 95–100 (2000).
Elbendary, A. et al. Transforming growth factor β1 can induce CIP1/WAF1 expression independent of the p53 pathway in ovarian cancer cells. Cell Growth Differ. 5, 1301–1307 (1994).
Datto, M. B. et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA 92, 5545–5549 (1995).
Li, C. Y., Suardet, L. & Little, J. B. Potential role of WAF1/Cip1/p21 as a mediator of TGF-β cytoinhibitory effect. J. Biol. Chem. 270, 4971–4974 (1995).
van de Wetering, M. et al. The β-catenin–TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Chen, T. et al. Clusterin-mediated apoptosis is regulated by adenomatous polyposis coli and is p21 dependent but p53 independent. Cancer Res. 64, 7412–7419 (2004).
Deng, C., Zhang, P., Harper, J. W., Elledge, S. J. & Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 (1995).
Martins, C. P. & Berns, A. Loss of p27Kip1 but not p21Cip1 decreases survival and synergizes with MYC in murine lymphomagenesis. EMBO J. 21, 3739–3748 (2002).
Martin-Caballero, J., Flores, J. M., Garcia-Palencia, P. & Serrano, M. Tumor susceptibility of p21Waf1/Cip1-deficient mice. Cancer Res. 61, 6234–6238 (2001). Shows that Cdkn1a−/− mice are resistant to developing lymphomas in response to γ-irradiation.
Yang, W. C. et al. Targeted inactivation of the p21WAF1/cip1 gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res. 61, 565–569 (2001).
Adnane, J. et al. Loss of p21WAF1/CIP1 accelerates Ras oncogenesis in a transgenic/knockout mammary cancer model. Oncogene 19, 5338–5347 (2000).
Bearss, D. J., Lee, R. J., Troyer, D. A., Pestell, R. G. & Windle, J. J. Differential effects of p21WAF1/CIP1 deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res. 62, 2077–2084 (2002).
Topley, G. I., Okuyama, R., Gonzales, J. G., Conti, C. & Dotto, G. P. p21WAF1/Cip1 functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc. Natl Acad. Sci. USA 96, 9089–9094 (1999).
Weinberg, W. C. et al. Genetic deletion of p21WAF1 enhances papilloma formation but not malignant conversion in experimental mouse skin carcinogenesis. Cancer Res. 59, 2050–2054 (1999).
Poole, A. J., Heap, D., Carroll, R. E. & Tyner, A. L. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene 23, 8128–8134 (2004).
Foster, K. W. et al. Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ. 10, 423–434 (1999). The first study to identify KLF4 as a potential oncogene.
Suzuki, T. et al. New genes involved in cancer identified by retroviral tagging. Nature Genet. 32, 166–174 (2002).
Foster, K. W. et al. Induction of KLF4 in basal keratinocytes blocks the proliferation–differentiation switch and initiates squamous epithelial dysplasia. Oncogene 24, 1491–1500 (2005). Shows that KLF4 overexpression in the squamous compartment of mice induces dysplasia that resemble squamous cell carcinomas in situ.
Foster, K. W. et al. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 60, 6488–6495 (2000). References 56 and 59 report that KLF4 is overexpressed in squamous cell carcinoma and breast cancer.
Pandya, A. Y. et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin. Cancer Res. 10, 2709–2719 (2004).
Raman, V. et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature 405, 974–978 (2000).
Yoon, H. S., Chen, X. & Yang, V. W. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J. Biol. Chem. 278, 2101–2105 (2003).
Rubinstein, M. et al. Transcriptional activation of the insulin-like growth factor I receptor gene by the Kruppel-like factor 6 (KLF6) tumor suppressor protein: potential interactions between KLF6 and p53. Endocrinology 145, 3769–3777 (2004).
Dang, D. T., Zhao, W., Mahatan, C. S., Geiman, D. E. & Yang, V. W. Opposing effects of Kruppel-like factor 4 (gut-enriched Kruppel-like factor) and Kruppel-like factor 5 (intestinal-enriched Kruppel-like factor) on the promoter of the Kruppel-like factor 4 gene. Nucleic Acids Res. 30, 2736–2741 (2002).
Liu, Y., Sinha, S. & Owens, G. A transforming growth factor-β control element required for SM α-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J. Biol. Chem. 278, 48004–48011 (2003).
Piccinni, S. A. et al. Kruppel-like factors regulate the Lama1 gene encoding the laminin α1 chain. J. Biol. Chem. 279, 9103–9114 (2004).
Narla, G. et al. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 65, 1213–1222 (2005).
Narla, G. et al. Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread. Cancer Res. 65, 5761–5768 (2005).
Land, H., Parada, L. F. & Weinberg, R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304, 596–602 (1983).
Wagner, A. J., Meyers, C., Laimins, L. A. & Hay, N. c-Myc induces the expression and activity of ornithine decarboxylase. Cell Growth Differ. 4, 879–883 (1993).
Bello-Fernandez, C., Packham, G. & Cleveland, J. L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl Acad. Sci. USA 90, 7804–7808 (1993).
Reisman, D., Elkind, N. B., Roy, B., Beamon, J. & Rotter, V. c-Myc trans-activates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 4, 57–65 (1993).
Chen, Z. Y., Shie, J. L. & Tseng, C. C. Gut-enriched Kruppel-like factor represses ornithine decarboxylase gene expression and functions as checkpoint regulator in colonic cancer cells. J. Biol. Chem. 277, 46831–46839 (2002).
Herold, S. et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol. Cell 10, 509–521 (2002).
Seoane, J., Le, H. V. & Massague, J. Myc suppression of the p21Cip1 Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 (2002).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Keblusek, P. et al. The adenoviral E1A oncoproteins interfere with the growth-inhibiting effect of the cdk-inhibitor p21CIP1/WAF1. J. Gen. Virol. 80, 381–390 (1999).
Whyte, P. et al. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature 334, 124–129 (1988).
Arany, Z., Newsome, D., Oldread, E., Livingston, D. M. & Eckner, R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature 374, 81–84 (1995).
Eckner, R. et al. Molecular cloning and functional analysis of the adenovirus E1A- associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8, 869–884 (1994).
Geiman, D. E., Ton-That, H., Johnson, J. M. & Yang, V. W. Transactivation and growth suppression by the gut-enriched Kruppel-like factor (Kruppel-like factor 4) are dependent on acidic amino acid residues and protein–protein interaction. Nucleic Acids Res. 28, 1106–1113 (2000).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Lowe, S. W., Jacks, T., Housman, D. E. & Ruley, H. E. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc. Natl Acad. Sci. USA 91, 2026–2030 (1994).
Lowe, S. W. & Ruley, H. E. Stabilization of the p53 tumor suppressor is induced by adenovirus-5 e1a and accompanies apoptosis. Genes Dev. 7, 535–545 (1993).
Debbas, M. & White, E. Wild-type p53 mediates apoptosis by e1a, which is inhibited by e1b. Genes Dev. 7, 546–554 (1993).
LaBaer, J. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
Cheng, M. et al. The p21Cip1 and p27Kip1 CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18, 1571–1583 (1999).
Pei, X. H. & Xiong, Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene 24, 2787–2795 (2005).
Diehl, J. A. & Sherr, C. J. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol. Cell. Biol. 17, 7362–7374 (1997).
Besson, A., Assoian, R. K. & Roberts, J. M. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nature Rev. Cancer 4, 948–955 (2004).
Shim, J., Lee, H., Park, J., Kim, H. & Choi, E. J. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature 381, 804–806 (1996).
Suzuki, A., Tsutomi, Y., Akahane, K., Araki, T. & Miura, M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 17, 931–939 (1998).
Asada, M. et al. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J. 18, 1223–1234 (1999). Reports that cytoplasmic p21 has anti-apoptotic functions.
Huang, S. et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21Cip1. Mol. Cell 11, 1491–1501 (2003).
Winters, Z. E. et al. Subcellular localisation of cyclin B, Cdc2 and p21WAF1/CIP1 in breast cancer. Association with prognosis. Eur. J. Cancer 37, 2405–2412 (2001).
Winters, Z. E., Leek, R. D., Bradburn, M. J., Norbury, C. J. & Harris, A. L. Cytoplasmic p21WAF1/CIP1 expression is correlated with HER-2/ neu in breast cancer and is an independent predictor of prognosis. Breast Cancer Res. 5, R242–R249 (2003).
Collins, N. L. et al. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol. Cell. Biol. 25, 5282–5291 (2005).
Wang, Y. A., Elson, A. & Leder, P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc. Natl Acad. Sci. USA 94, 14590–14595 (1997). Shows that p21-deficiency reduces lymphomagenesis in Atm−/− mice.
Waldman, T., Lengauer, C., Kinzler, K. W. & Vogelstein, B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381, 713–716 (1996).
Fan, S. et al. Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene 14, 2127–2136 (1997).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005). References 101 and 102 report that human pre-malignant lesions show DNA damage foci in situ.
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Kim, C. F. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835 (2005).
Zhou, B. P. et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biol. 3, 245–252 (2001). Shows that ERBB2 causes AKT-mediated p21 phosphorylation, which results in its cytoplasmic retention.
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Chi, X. Z. et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21WAF1/Cip1 expression in cooperation with transforming growth factor β-activated SMAD. Mol. Cell. Biol. 25, 8097–8107 (2005).
Li, Q. L. et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109, 113–124 (2002).
Zavadil, J., Cermak, L., Soto-Nieves, N. & Bottinger, E. P. Integration of TGF-β–Smad and Jagged1–Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 23, 1155–1165. (2004).
King, K. E., Iyemere, V. P., Weissberg, P. L. & Shanahan, C. M. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor β1 in the regulation of vascular smooth muscle cell phenotype. J. Biol. Chem. 278, 11661–11669 (2003).
Bachman, K. E. et al. p21WAF1/CIP1 mediates the growth response to TGF-β in human epithelial cells. Cancer Biol. Ther. 3, 221–225 (2004).
Kim, Y. et al. Transcriptional activation of transforming growth factor β1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J. Biol. Chem. 273, 33750–33758 (1998).
Shindo, T. et al. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nature Med. 8, 856–863 (2002).
Ellenrieder, V. et al. KLF11 mediates a critical mechanism in TGF-β signaling that is inactivated by Erk–MAPK in pancreatic cancer cells. Gastroenterology 127, 607–620 (2004).
da Costa, L. T. et al. CDX2 is mutated in a colorectal cancer with normal APC–β-catenin signaling. Oncogene 18, 5010–5014 (1999).
Li, D. et al. KLF6 promotes preadipocyte differentiation through histone deacetylase 3 (HDAC3)-dependent repression of Dlk1. J. Biol. Chem. 280, 26941–26952 (2005).
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Jensen, J. et al. Control of endodermal endocrine development by Hes-1. Nature Genet. 24, 36–44 (2000).
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 (2001).
Fre, S. et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 (2005).
Andreu, P. et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132, 1443–1451 (2005).
Herrera, R., Mäkelä, T. P. & Weinberg, R. A. TGFβ-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol. Biol. Cell 7, 1335–1342 (1996).
Zhang, H. S., Postigo, A. A. & Dean, D. C. Active transcriptional repression by the Rb–E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell 97, 53–61 (1999).
Devgan, V., Mammucari, C., Millar, S. E., Brisken, C. & Dotto, G. P. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 19, 1485–1495 (2005).
Barbacid, M. ras genes. Annu Rev. Biochem 56, 779–827 (1987).
Malumbres, M. & Barbacid, M. RAS oncogenes: the first 30 years. Nature Rev. Cancer 3, 459–465 (2003).
Zhang, Z. et al. Wildtype Kras2 can inhibit lung carcinogenesis in mice. Nature Genet. 29, 25–33 (2001).
Takahashi, C. et al. N-ras loss induces metastatic conversion of Rb-deficient neuroendocrine thyroid tumor. Nature Genet. (in the press).
Coleman, M. L., Marshall, C. J. & Olson, M. F. Ras promotes p21Waf1/Cip1 protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. EMBO J. 22, 2036–2046 (2003).
Kivinen, L. et al. Ras induces p21Cip1/Waf1 cyclin kinase inhibitor transcriptionally through Sp1-binding sites. Oncogene 18, 6252–6261 (1999).
Mack, F. A., Patel, J. H., Biju, M. P., Haase, V. H. & Simon, M. C. Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol. Cell. Biol. 25, 4565–4578 (2005).
Bardeesy, N. et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419, 162–167 (2002). Shows that LKB1 deficiency causes the formation of polyps in mice, whereas LKB1-deficient cells are resistant to transformation in vitro.
Tiainen, M., Vaahtomeri, K., Ylikorkala, A. & Makela, T. P. Growth arrest by the LKB1 tumor suppressor: induction of p21WAF1/CIP1. Hum. Mol. Genet. 11, 1497–1504 (2002).
Shie, J. L., Chen, Z. Y., Fu, M., Pestell, R. G. & Tseng, C. C. Gut-enriched Kruppel-like factor represses cyclin D1 promoter activity through Sp1 motif. Nucleic Acids Res. 28, 2969–2976 (2000).
Wang, H., Yang, L., Jamaluddin, M. S. & Boyd, D. D. The Kruppel-like KLF4 transcription factor, a novel regulator of urokinase receptor expression, drives synthesis of this binding site in colonic crypt luminal surface epithelial cells. J. Biol. Chem. 279, 22674–22683 (2004).
Miller, K. A. et al. Kruppel-like factor 4 regulates lamininα 3A expression in mammary epithelial cells. J. Biol. Chem. 276, 42863–42868 (2001).
Yoon, H. S. & Yang, V. W. Requirement of Kruppel-like factor 4 in preventing entry into mitosis following DNA damage. J. Biol. Chem. 279, 5035–5041 (2004).
Higaki, Y. et al. Synergistic activation of the rat lamininγ1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1. Nucleic Acids Res. 30, 2270–2279 (2002).
Noti, J. D., Johnson, A. K. & Dillon, J. D. The leukocyte integrin gene CD11d is repressed by gut-enriched Kruppel-like factor 4 in myeloid cells. J. Biol. Chem. 280, 3449–3457 (2005).
Hinnebusch, B. F. et al. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G23–G30 (2004).
Brembeck, F. H. & Rustgi, A. K. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J. Biol. Chem. 275, 28230–28239 (2000).
Jenkins, T. D., Opitz, O. G., Okano, J. & Rustgi, A. K. Transactivation of the human keratin 4 and Epstein–Barr virus ED-L2 promoters by gut-enriched Kruppel-like factor. J. Biol. Chem. 273, 10747–10754 (1998).
Ai, W., Liu, Y., Langlois, M. & Wang, T. C. Kruppel-like factor 4 (KLF4) represses histidine decarboxylase gene expression through an upstream Sp1 site and downstream gastrin responsive elements. J. Biol. Chem. 279, 8684–8693 (2004).
Saifudeen, Z., Dipp, S., Fan, H. & El-Dahr, S. S. Combinatorial control of the bradykinin B2 receptor promoter by p53, CREB, KLF-4, and CBP: implications for terminal nephron differentiation. Am. J. Physiol. Renal Physiol. 288, F899–F909 (2005).
Zhang, W., Shields, J. M., Sogawa, K., Fujii-Kuriyama, Y. & Yang, V. W. The gut-enriched Kruppel-like factor suppresses the activity of the CYP1A1 promoter in an Sp1-dependent fashion. J. Biol. Chem. 273, 17917–17925 (1998).
Mao, Z. et al. Transcriptional regulation of A33 antigen expression by gut-enriched Kruppel-like factor. Oncogene 22, 4434–4443 (2003).
Adam, P. J., Regan, C. P., Hautmann, M. B. & Owens, G. K. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor β control element required for expression of the smooth muscle cell differentiation marker SM22α in vivo. J. Biol. Chem. 275, 37798–37806 (2000).
Shields, J. M. & Yang, V. W. Identification of the DNA sequence that interacts with the gut-enriched Kruppel-like factor. Nucleic Acids Res. 26, 796–802 (1998).
Bahl, R. et al. Novel polymorphism in p21waf1/cip1 cyclin dependent kinase inhibitor gene: association with human esophageal cancer. Oncogene 19, 323–328 (2000).
Balbin, M. et al. Functional analysis of a p21WAF1,CIP1,SDI1 mutant (Arg94→ Trp) identified in a human breast carcinoma. Evidence that the mutation impairs the ability of p21 to inhibit cyclin-dependent kinases. J. Biol. Chem. 271, 15782–15786 (1996).
Ralhan, R., Agarwal, S., Mathur, M., Wasylyk, B. & Srivastava, A. Association between polymorphism in p21Waf1/Cip1 cyclin-dependent kinase inhibitor gene and human oral cancer. Clin. Cancer Res. 6, 2440–2447 (2000).
Biankin, A. V. et al. Overexpression of p21WAF1/CIP1 is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 61, 8830–8837 (2001).
Carnero, A. & Beach, D. H. Absence of p21WAF1 cooperates with c-myc in bypassing Ras-induced senescence and enhances oncogenic cooperation. Oncogene 23, 6006–6011 (2004).
Kawata, S., Ariumi, Y. & Shimotohno, K. p21Waf1/Cip1/Sdi1 prevents apoptosis as well as stimulates growth in cells transformed or immortalized by human T-cell leukemia virus type 1-encoded tax. J. Virol. 77, 7291–7299 (2003).
Villanueva, A. et al. Disruption of the antiproliferative TGF-β signaling pathways in human pancreatic cancer cells. Oncogene 17, 1969–1978 (1998).
Hahn, S. A. et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271, 350–353 (1996).
Schutte, M. et al. DPC4 gene in various tumor types. Cancer Res. 56, 2527–2530 (1996).
Goggins, M. et al. Genetic alterations of the transforming growth factor β receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 58, 5329–5332 (1998).
Markowitz, S. et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338 (1995).
Derynck, R. et al. Synthesis of messenger RNAs for transforming growth factors α and β and the epidermal growth factor receptor by human tumors. Cancer Res. 47, 707–712 (1987).
Crawford, S. E. et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93, 1159–1170 (1998).
Xu, X. et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19, 1868–1874 (2000).
Zhu, Y., Richardson, J. A., Parada, L. F. & Graff, J. M. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94, 703–714 (1998).
Oft, M., Heider, K. H. & Beug, H. TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8, 1243–1252 (1998).
Yin, J. J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Wotton, S. F. et al. RUNX1 transformation of primary embryonic fibroblasts is revealed in the absence of p53. Oncogene 23, 5476–5486 (2004).
Song, W. J. et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nature Genet. 23, 166–175 (1999).
Niini, T., Kanerva, J., Vettenranta, K., Saarinen-Pihkala, U. M. & Knuutila, S. AML1 gene amplification: a novel finding in childhood acute lymphoblastic leukemia. Haematologica 85, 362–366 (2000).
Harewood, L. et al. Amplification of AML1 on a duplicated chromosome 21 in acute lymphoblastic leukemia: a study of 20 cases. Leukemia 17, 547–553 (2003).
Robinson, H. M. et al. Amplification of AML1 in acute lymphoblastic leukemia is associated with a poor outcome. Leukemia 17, 2249–2250 (2003).
Vaillant, F. et al. A full-length Cbfa1 gene product perturbs T-cell development and promotes lymphomagenesis in synergy with myc. Oncogene 18, 7124–7134 (1999).
Lund, A. H. et al. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nature Genet. 32, 160–165 (2002).
Spender, L. C., Whiteman, H. J., Karstegl, C. E. & Farrell, P. J. Transcriptional cross-regulation of RUNX1 by RUNX3 in human B cells. Oncogene 24, 1873–1881 (2005).
Thelu, J., Rossio, P. & Favier, B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2, 7 (2002).
Reynolds, T. C., Smith, S. D. & Sklar, J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the β T cell receptor gene in human lymphoblastic neoplasms. Cell 50, 107–117 (1987).
Ellisen, L. W. et al. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 (1991).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature Genet. 33, 416–421 (2003).
Saito, M. et al. Amplification of the E2F1 transcription factor gene in the HEL erythroleukemia cell line. Genomics 25, 130–138 (1995).
Feber, A. et al. Amplification and overexpression of E2F3 in human bladder cancer. Oncogene 23, 1627–1630 (2004).
Yamasaki, L. et al. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85, 537–548 (1996).
Zhu, J. W. et al. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol. Cell. Biol. 21, 8547–8564 (2001).
Pierce, A. M., Fisher, S. M., Conti, C. J. & Johnson, D. G. Deregulated expression of E2F1 induces hyperplasia and cooperates with ras in skin tumor development. Oncogene 16, 1267–1276 (1998).
Yamasaki, L. et al. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1+/− mice. Nature. Genet. 18, 360–364 (1998).
Wu, X. & Levine, A. J. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl Acad. Sci. USA 91, 3602–3606 (1994).
Shan, B. & Lee, W. H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol. Cell. Biol. 14, 8166–8173 (1994).
Qin, X. Q., Livingston, D. M., Kaelin, W. G. Jr & Adams, P. D. Deregulated transcription factor E2F-1 expression leads to S- phase entry and p53-mediated apoptosis. Proc. Natl Acad. Sci. USA 91, 10918–10922 (1994).
Dimri, G. P., Itahana, K., Acosta, M. & Campisi, J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14ARF tumor suppressor. Mol. Cell. Biol. 20, 273–285 (2000).
Singh, P., Wong, S. H. & Hong, W. Overexpression of E2F-1 in rat embryo fibroblasts leads to neoplastic transformation. EMBO J. 13, 3329–3338 (1994).
Johnson, D. G., Cress, W. D., Jakoi, L. & Nevins, J. R. Oncogenic capacity of the E2F1 gene. Proc. Natl Acad. Sci. USA 91, 12823–12827 (1994).
Xu, G., Livingston, D. M. & Krek, W. Multiple members of the E2F transcription factor family are the products of oncogenes. Proc. Natl Acad. Sci. USA 92, 1357–1361 (1995).
Bar-Sagi, D. & Feramisco, J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42, 841–848 (1985).
Ruley, H. E. Adenovirus region E1A enables viral and cellular transforming genes to transform primary cells in cultures. Nature 304, 602–606 (1983).
Newbold, R. F. & Overell, R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature 304, 648–651 (1983).
Jenne, D. E. et al. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nature Genet. 18, 38–43 (1998).
Hemminki, A. et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391, 184–187 (1998).
Jishage, K. et al. Role of Lkb1, the causative gene of Peutz–Jegher's syndrome, in embryogenesis and polyposis. Proc. Natl Acad. Sci. USA 99, 8903–8908 (2002).
Miyoshi, H. et al. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 62, 2261–2266 (2002).
Tiainen, M., Ylikorkala, A. & Makela, T. P. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc. Natl Acad. Sci. USA 96, 9248–9251 (1999).
Latif, F. et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993).
Gnarra, J. R. et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nature Genet. 7, 85–90 (1994).
Foster, K. et al. Somatic mutations of the von Hippel–Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum. Mol. Genet 3, 2169–2173 (1994).
Lee, J. Y. et al. Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 58, 504–508 (1998).
Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc. Natl Acad. Sci. USA 98, 1583–1588 (2001).
Ma, W. et al. Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res. 63, 5320–5328 (2003).
Mack, F. A. et al. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 3, 75–88 (2003).
Pause, A., Lee, S., Lonergan, K. M. & Klausner, R. D. The von Hippel–Lindau tumor suppressor gene is required for cell cycle exit upon serum withdrawal. Proc. Natl Acad. Sci. USA 95, 993–998 (1998).
Acknowledgements
We are grateful to M. Ewen and A. Rustgi for sharing unpublished data, S. Friedman for helpful suggestions and M. van Lohuizen for critical reading of the manuscript. B.D.R. and D.S.P. are supported by grants from the Dutch Cancer Society (KWF) and the Netherlands Organization for Scientific Research (NWO), and D.S.P. is an EMBO young investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Epithelial to mesenchymal transition
-
Loss of epithelial cell polarity and cell–cell contacts with repression of epithelial markers, often associated with acquisition of fibroblastic characteristics.
Rights and permissions
About this article
Cite this article
Rowland, B., Peeper, D. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer 6, 11–23 (2006). https://doi.org/10.1038/nrc1780
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1780
This article is cited by
-
Expression of stem cell markers SALL4, LIN28A, and KLF4 in ameloblastoma
Diagnostic Pathology (2023)
-
SET8 is a novel negative regulator of TGF-β signaling in a methylation-independent manner
Scientific Reports (2023)
-
KLF4 transcription factor in tumorigenesis
Cell Death Discovery (2023)
-
Therapeutic Targeting of Krüppel-Like Factor 4 and Its Pharmacological Potential in Parkinson’s Disease: a Comprehensive Review
Molecular Neurobiology (2023)
-
Application of Induced Pluripotent Stem Cells in Malignant Solid Tumors
Stem Cell Reviews and Reports (2023)