Abstract
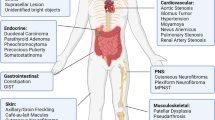
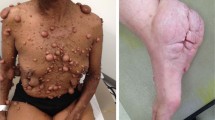
Neurofibromatosis type 1 (NF1) is a common genetic condition in which affected individuals develop benign and malignant nervous system tumours. Genetically engineered mouse (GEM) models of these NF1-associated nervous system tumours recapitulate several of the unique clinical aspects of the disease. Moreover, these Nf1 GEM models allow for a direct examination of the earliest stages of tumour evolution, including the contributions that Nf1+/− cellular elements and cooperating genetic changes make to facilitate the transition from the pre-neoplastic to the neoplastic state and, in some cases, to promote malignant progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedman, J. M., Gutmann, D. H., MacCollin, M. M. & Riccardi, V. M. (eds) Neurofibromatosis: Phenotype, Natural History & Pathogenesis (Johns Hopkins Univ. Press, Baltimore, Maryland, 1999).
Knudson, A. G. Two genetic hits (more or less) to cancer. Nature Rev. Cancer 1, 157–162 (2001).
Sherman, L. S., Atit, R., Rosenbaum, T., Cox, A. D. & Ratner, N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J. Biol. Chem. 275, 30740–30745 (2000).
Perry, A., Roth, K. A., Banerjee, R., Fuller, C. E. & Gutmann, D. H. NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am. J. Pathol. 159, 57–61 (2001).
Rutkowski, J. L., Wu, K., Gutmann, D. H., Boyer, P. J. & Legius, E. Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum. Mol. Genet. 9, 1059–1066 (2000).
Ferner, R. E. & Gutmann, D. H. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 62, 1573–1577 (2002).
King, A. A., Debaun, M. R., Riccardi, V. M. & Gutmann, D. H. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am. J. Med. Genet. 93, 388–392 (2000).
Korf, B. R. Plexiform neurofibromas. Am. J. Med. Genet. 89, 31–37 (1999).
Watson, M. A. et al. Gene expression profiling reveals unique molecular subtypes of Neurofibromatosis type I-associated and sporadic malignant peripheral nerve sheath tumors. Brain Pathol. 14, 297–303 (2004).
Perry, A. et al. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J. Neuropathol. Exp. Neurol. 61, 702–709 (2002).
Kourea, H. P., Cordon-Cardo, C., Dudas, M., Leung, D. & Woodruff, J. M. Expression of p27kip and other cell cycle regulators in malignant peripheral nerve sheath tumors and neurofibromas: the emerging role of p27kip in malignant transformation of neurofibromas. Am. J. Pathol. 155, 1885–1891 (1999).
Kourea, H. P., Orlow, I., Scheithauer, B. W., Cordon-Cardo, C. & Woodruff, J. M. Deletions of the INK4A gene occur in malignant peripheral nerve sheath tumors but not in neurofibromas. Am. J. Pathol. 155, 1855–1860 (1999).
Nielsen, G. P. et al. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am. J. Pathol. 155, 1879–1884 (1999).
Listernick, R., Charrow, J. & Gutmann, D. H. Intracranial gliomas in neurofibromatosis type 1. Am. J. Med. Genet. 89, 38–44 (1999).
King, A., Listernick, R., Charrow, J., Piersall, L. & Gutmann, D. H. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am. J. Med. Genet. A 122, 95–99 (2003).
Thiagalingam, S., Flaherty, M., Billson, F. & North, K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology 111, 568–577 (2004).
Parsa, C. F. et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch. Ophthalmol. 119, 516–529 (2001).
Gutmann, D. H., Donahoe, J., Brown, T., James, C. D. & Perry, A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol. Appl. Neurobiol. 26, 361–367 (2000).
Li, J., Perry, A., James, C. D. & Gutmann, D. H. Cancer-related gene expression profiles in NF1-associated pilocytic astrocytomas. Neurology 56, 885–890 (2001).
Wimmer, K., Eckart, M., Meyer-Puttlitz, B., Fonatsch, C. & Pietsch, T. Mutational and expression analysis of the NF1 gene argues against a role as tumor suppressor in sporadic pilocytic astrocytomas. J. Neuropathol. Exp. Neurol. 61, 896–902 (2002).
Gutmann, D. H. et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 59, 759–761 (2002).
Gutmann, D. H. et al. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology 61, 1397–1400 (2003).
Wallace, M. R. et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 249, 181–186 (1990).
Viskochil, D. et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 62, 187–192 (1990).
Gutmann, D. H., Zhang, Y. & Hirbe, A. Developmental regulation of a neuron-specific neurofibromatosis 1 isoform. Ann. Neurol. 46, 777–782 (1999).
Costa, R. M. et al. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nature Genet. 27, 399–405 (2001).
Xu, G. F. et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62, 599–608 (1990).
Xu, G. F. et al. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell 63, 835–841 (1990).
Martin, G. A. et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell 63, 843–849 (1990).
Ballester, R. et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell 63, 851–859 (1990).
Basu, T. N. et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356, 713–715 (1992).
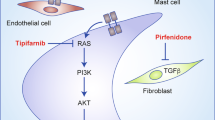
Bollag, G. et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nature Genet. 12, 144–148 (1996).
DeClue, J. E. et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69, 265–273 (1992).
Kim, H. A., Rosenbaum, T., Marchionni, M. A., Ratner, N. & DeClue, J. E. Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene 11, 325–335 (1995).
Hiatt, K. K., Ingram, D. A., Zhang, Y., Bollag, G. & Clapp, D. W. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1−/− cells. J. Biol. Chem. 276, 7240–7245 (2001).
Cichowski, K., Santiago, S., Jardim, M., Johnson, B. W. & Jacks, T. Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev. 17, 449–454 (2003).
Tang, Y. et al. A role for Pak protein kinases in Schwann cell transformation. Proc. Natl Acad. Sci. USA 95, 5139–5144 (1998).
Ingram, D. A. et al. Hyperactivation of p21ras and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J. Exp. Med. 194, 57–69 (2001).
Yang, F. C. et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J. Clin. Invest. 112, 1851–1861 (2003).
Donovan, S., See, W., Bonifas, J., Stokoe, D. & Shannon, K. M. Hyperactivation of protein kinase B and ERK have discrete effects on survival, proliferation, and cytokine expression in Nf1-deficient myeloid cells. Cancer Cell 2, 507–514 (2002).
Lau, N. et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J. Neuropathol. Exp. Neurol. 59, 759–767 (2000).
Zhang, Y. Y. et al. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J. Exp. Med. 187, 1893–1902 (1998).
Bajenaru, M. L. et al. Neurofibromatosis 1 (NF1) heterozygosity results in a cell-autonomous growth advantage for astrocytes. Glia 33, 314–323 (2001).
Gutmann, D. H. et al. Heterozygosity for the neurofibromatosis 1 (NF1) tumor suppressor results in abnormalities in cell attachment, spreading and motility in astrocytes. Hum. Mol. Genet. 10, 3009–3016 (2001).
The, I. et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 276, 791–794 (1997).
Guo, H. F., The, I., Hannan, F., Bernards, A. & Zhong, Y. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science 276, 795–798 (1997).
Johnson, M. R., Look, A. T., DeClue, J. E., Valentine, M. B. & Lowy, D. R. Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP. Ras. Proc. Natl Acad. Sci. USA 90, 5539–5543 (1993).
Gutmann, D. H. et al. Analysis of the neurofibromatosis type 1 (NF1) GAP-related domain by site-directed mutagenesis. Oncogene 8, 761–769 (1993).
Waschek, J. A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 24, 14–23 (2002).
Dasgupta, B., Dugan, L. L. & Gutmann, D. H. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J. Neurosci. 23, 8949–8954 (2003).
Kim, H. A., Ratner, N., Roberts, T. M. & Stiles, C. D. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J. Neurosci. 21, 1110–1116 (2001).
Houslay, M. D. & Baillie, G. S. The role of ERK2 docking and phosphorylation of PDE4 cAMP phosphodiesterase isoforms in mediating cross-talk between the cAMP and ERK signalling pathways. Biochem. Soc. Trans. 31, 1186–1190 (2003).
Jacks, T. et al. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genet. 7, 353–361 (1994).
Zhu, Y. et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15, 859–876 (2001).
Zhu, Y., Ghosh, P., Charnay, P., Burns, D. K. & Parada, L. F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 296, 920–922 (2002).
Kim, H. A., Ling, B. & Ratner, N. Nf1-deficient mouse Schwann cells are angiogenic and invasive and can be induced to hyperproliferate: reversion of some phenotypes by an inhibitor of farnesyl protein transferase. Mol. Cell. Biol. 17, 862–872 (1997).
Le, D. T. et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood 103, 4243–4250 (2004).
Atit, R. P., Crowe, M. J., Greenhalgh, D. G., Wenstrup, R. J. & Ratner, N. The Nf1 tumor suppressor regulates mouse skin wound healing, fibroblast proliferation, and collagen deposited by fibroblasts. J. Invest. Dermatol. 112, 835–842 (1999).
Bajenaru, M. L. et al. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol. Cell. Biol. 22, 5100–5113 (2002).
Bajenaru, M. L. et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 63, 8573–8577 (2003).
Bajenaru, M. L., Garbow, J. R., Perry, A., Hernandez, M. R. & Gutmann, D. H. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann. Neurol. 57, 119–127 (2005).
Reilly, K. M., Loisel, D. A., Bronson, R. T., McLaughlin, M. E. & Jacks, T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nature Genet. 26, 109–113 (2000).
Ridley, A. J., Paterson, H. F., Noble, M. & Land, H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 7, 1635–1645 (1988).
Dasgupta, B., Li, W., Perry, A., Gutmann, D. H. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 65, 236–245 (2005).
King, D., Yang, G., Thompson, M. A. & Hiebert, S. W. Loss of neurofibromatosis-1 and p19ARF cooperate to induce a multiple tumor phenotype. Oncogene 21, 4978–4982 (2002).
Reilly, K. M. et al. Susceptibility to astrocytoma in mice mutant for Nf1 and Trp53 is linked to chromosome 11 and subject to epigenetic effects. Proc. Natl Acad. Sci. USA 101, 13008–13013 (2004).
Kim, J. Y. et al. Medulloblastoma tumorigenesis diverges from cerebellar granule cell differentiation in patched heterozygous mice. Dev. Biol. 263, 50–66 (2003).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Woerner, B. et al. in Ninth Annual Meeting of the Society for Neuro-Oncology (ed. Bigner, D. D.) 323 (Duke Univ. Press, Toronto, Ontario Canada, 2004).
Dasgupta, B., Yi, Y., Chen, D. Y., Weber, J. D. & Gutmann, D. H. Proteomic analysis reveals hyperactivation of the mTOR pathway in NF1-associated human and mouse brain tumors. Cancer Res. 65, 2755–2760 (2005).
Oliver T. G. et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development 132, 2425–2439 (2005)
Acknowledgements
The authors thank our Neurofibromatosis Center Group at Washington University, especially A. Perry, J. Weber, M. Watson and J. Garbow, as well as R. Wechsler-Reya for critical reading of the manuscript. D.H.G. is supported by grants from the Department of Defense, National Institute of Neurological Disorders and Strokes, and James S. McDonnell Foundation. J.R. is a scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine and receives additional support from the National Institute of Child Health and Human Development, The American Cancer Society and Hope Street Kids. We also acknowledge the generous support from Schnuck Markets, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
OMIM
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Rubin, J., Gutmann, D. Neurofibromatosis type 1 — a model for nervous system tumour formation?. Nat Rev Cancer 5, 557–564 (2005). https://doi.org/10.1038/nrc1653
Issue Date:
DOI: https://doi.org/10.1038/nrc1653
This article is cited by
-
Isolated Neurofibroma of the Sigmoid Colon: a Case Report and Review of the Literature
Journal of Gastrointestinal Cancer (2018)
-
Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors
Oncogene (2017)
-
Cancer of the Peripheral Nerve in Neurofibromatosis Type 1
Neurotherapeutics (2017)
-
Hyperactive interneurons impair learning in a neurofibromatosis model
Nature Neuroscience (2009)