Key Points
-
Nuclear-cytoplasmic transport of oncogenes and tumour suppressors is disrupted in cancer cells. Transport can be regulated by modification of the cargo, at the level of the transport machinery or at the level of the nuclear-pore complex (NPC). Modifying the nuclearcytoplasmic transport activity might block tumorigenesis.
-
Karyopherin-α and karyopherin-β transport cargoes that contain nuclear localization signals (NLSs) into the nucleus through the NPC. CRM1 is the transport receptor that exports nuclear export signal (NES)-containing proteins out of the nucleus.
-
The RanGTP/GDP gradient across the nuclear envelope promotes the direction of transport.
-
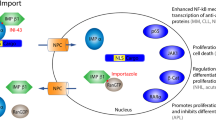
Hyper-active forms of AKT can lead to the mislocalization and therefore inactivation of proteins such as the FOXO transcription factors and p27 cell-cycle inhibitor.
-
Wild-type p53 is mislocalized to the cytoplasm in many cancers, possibly due, in part, to association with the cytoplasmic anchor protein PARC.
-
Stable, nuclear β-catenin is a hallmark of colon cancer cells. Experiments have implicated inefficient export by a mutant form of APC as a possible cause of nuclear β-catenin.
-
Altered expression of nuclear transport factors, such as karyopherins and nucleoporins, have been implicated in tumorigenesis.
-
Leptomycin B is a potent and specific covalent inhibitor of CRM1 and can sequester proteins in the nucleus.
-
Visual, high-throughput and high-content small-molecule screens for compounds that re-direct mislocalized proteins to the correct cellular compartment might reveal novel anticancer agents.
Abstract
Nuclear-cytoplasmic transport, which occurs through special structures called nuclear pores, is an important aspect of normal cell function, and defects in this process have been detected in many different types of cancer cells. These defects can occur in the signal-transduction pathways that regulate the transfer of factors such as p53 and β-catenin in and out of the nucleus, or in the general nuclear import and export machinery itself. In some cases, nuclear transport factors are overproduced, whereas in others, chromosomal translocations disrupt the structural proteins that make up the nuclear pore, leading to cell transformation. How does disruption of nuclear-cytoplasmic transport promote transformation, and is this process a viable therapeutic target?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Akey, C. W. & Radermacher, M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J. Cell Biol. 122, 1–19 (1993).
Hinshaw, J. E., Carragher, B. O. & Milligan, R. A. Architecture and design of the nuclear pore complex. Cell 69, 1133–1141 (1992).
Goldberg, M. W. & Allen, T. D. High resolution scanning electron microscopy of the nuclear envelope: demonstration of a new, regular, fibrous lattice attached to the baskets of the nucleoplasmic face of the nuclear pores. J. Cell Biol. 119, 1429–1440 (1992).
Stoffler, D., Fahrenkrog, B. & Aebi, U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr. Opin. Cell Biol. 11, 391–401 (1999).
Miller, B. R. & Forbes, D. J. Purification of the vertebrate nuclear pore complex by biochemical criteria. Traffic 1, 941–951 (2000).
Cronshaw, J. M., Krutchinsky, A. N., Zhang, W., Chait, B. T. & Matunis, M. J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 158, 915–927 (2002).
Rout, M. P. & Aitchison, J. D. The nuclear pore complex as a transport machine. J. Biol. Chem. 276, 16593–16596 (2001).
Ryan, K. J. & Wente, S. R. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 12, 361–371 (2000).
Gorlich, D. & Kutay, U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660 (1999). Excellent review on nuclear transport factors and processes.
Mattaj, I. W. & Englmeier, L. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67, 265–306 (1998).
Strom, A. C. & Weis, K. Importin-β-like nuclear transport receptors. Genome Biol. 2, REVIEWS3008 (2001).
Izaurralde, E., Kutay, U., von Kobbe, C., Mattaj, I. W. & Gorlich, D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 16, 6535–6547 (1997).
Marelli, M., Dilworth, D. J., Wozniak, R. W. & Aitchison, J. D. The dynamics of karyopherin-mediated nuclear transport. Biochem. Cell Biol. 79, 603–612 (2001).
Uchida, S. et al. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol. Cell. Biol. 10, 577–584 (1990).
Feldherr, C. M. & Akin, D. Signal-mediated nuclear transport in proliferating and growth-arrested BALB/c 3T3 cells. J. Cell Biol. 115, 933–939 (1991).
Rayet, B. & Gelinas, C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18, 6938–6947 (1999).
Perkins, N. D. The Rel/NF-kappa B family: friend and foe. Trends Biochem. Sci. 25, 434–440 (2000).
Karin, M., Cao, Y., Greten, F. R. & Li, Z. W. NF-κβ in cancer: from innocent bystander to major culprit. Nature Rev. Cancer 2, 301–310 (2002).
Henkel, T. et al. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-κβ subunit. Cell 68, 1121–1133 (1992).
Beg, A. A. et al. I κβ interacts with the nuclear localization sequences of the subunits of NF-κβ: a mechanism for cytoplasmic retention. Genes Dev. 6, 1899–1913 (1992).
Ganchi, P. A., Sun, S. C., Greene, W. C. & Ballard, D. W. I κβ/MAD-3 masks the nuclear localization signal of NF-κβ p65 and requires the transactivation domain to inhibit NF-kappa B p65 DNA binding. Mol. Biol. Cell 3, 1339–1352 (1992).
Chen, L. F. & Greene, W. C. Regulation of distinct biological activities of the NF-κβ transcription factor complex by acetylation. J. Mol. Med. 81, 549–557 (2003).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
del Peso, L., Gonzalez, V. M., Hernandez, R., Barr, F. G. & Nunez, G. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene 18, 7328–7333 (1999).
Kops, G. J. P. L. et al. Direct control of the forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Rena, G., Guo, S., Cichy, S. C., Unterman, T. G. & Cohen, P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274, 17179–17183 (1999).
Takaishi, H. et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc. Natl Acad. Sci. USA 96, 11836–11841 (1999).
Tang, E. D., Nunez, G., Barr, F. G. & Guan, K. L. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274, 16741–16746 (1999).
Medema, R. H., Kops, G. J. P. L., Bos, J. L. & Burgering, B. M. T. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27KIP1. Nature 404, 782–787 (2000).
Nakamura, N. et al. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20, 8969–8982 (2000). FKHR or FOXO1a is mislocalized in PTEN -null cancer cells and reconstitution of FKHR to the nucleus results in cell-cycle arrest or apoptosis.
Brunet, A. et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156, 817–828 (2002).
Rena, G., Prescott, A. R., Guo, S., Cohen, P. & Unterman, T. G. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem. J. 354, 605–612 (2001).
Maehama, T. & Dixon, J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Vazquez, F. & Sellers, W. R. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signaling. Biochim. Biophys. Acta 1470, M21–M35 (2000).
Kondo, K. et al. PTEN/MMAC1/TEP1 mutations in human primary renal-cell carcinomas and renal carcinoma cell lines. Int. J. Cancer 91, 219–224 (2001).
Kong, D. et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nature Genet. 17, 143–144 (1997).
Wang, S. I. et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 57, 4183–4186 (1997).
Brunet, A. et al. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21, 952–965 (2001).
Mazumdar, A. & Kumar, R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 535, 6–10 (2003).
Rena, G. et al. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J. 21, 2263–2271 (2002).
Woods, Y. L. et al. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem. J. 355, 597–607 (2001).
Biggs, W. H., Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl Acad. Sci. USA 96, 7421–7426 (1999).
Brownawell, A. M., Kops, G. J., Macara, I. G. & Burgering, B. M. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell. Biol. 21, 3534–3546 (2001).
Ramaswamy, S. et al. Regulation of G1 progression by the PTEN tumor supressor protein is linked to inhibition of the phosphatidylinositol-3-kinase/Akt pathway. Proc. Natl Acad. Sci. USA 96, 2110–2115 (1999).
Blain, S. W. & Massague, J. Breast cancer banishes p27 from nucleus. Nature Med. 8, 1076–1078 (2002).
Liang, J. et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nature Med. 8, 1153–1160 (2002).
Shin, I. et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nature Med. 8, 1145–1152 (2002).
Viglietto, G. et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nature Med. 8, 1136–1144 (2002). References 46–48 show how p27 phosphorylation by Akt results in p27 nuclear export and cell proliferation.
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Ryan, K. M., Phillips, A. C. & Vousden, K. H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13, 332–337 (2001).
Shaulsky, G., Goldfinger, N., Ben-Ze'ev, A. & Rotter, V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol. Cell. Biol. 10, 6565–6577 (1990).
Liang, S. H., Hong, D. & Clarke, M. F. Cooperation of a single lysine mutation and a C-terminal domain in the cytoplasmic sequestration of the p53 protein. J. Biol. Chem. 273, 19817–19821 (1998).
Stommel, J. M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660–1672 (1999).
Zhang, Y. & Xiong, Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science 292, 1910–1915 (2001).
Tao, W. & Levine, A. J. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc. Natl Acad. Sci. USA 96, 3077–3080 (1999).
Geyer, R. K., Yu, Z. K. & Maki, C. G. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nature Cell Biol. 2, 569–573 (2000).
Boyd, S. D., Tsai, K. Y. & Jacks, T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nature Cell Biol. 2, 563–568 (2000).
Lohrum, M. A., Woods, D. B., Ludwig, R. L., Balint, E. & Vousden, K. H. C-terminal ubiquitination of p53 contributes to nuclear export. Mol. Cell. Biol. 21, 8521–8532 (2001).
Gu, J., Nie, L., Wiederschain, D. & Yuan, Z. M. Identification of p53 sequence elements that are required for MDM2-mediated nuclear export. Mol. Cell. Biol. 21, 8533–8546 (2001).
Harris, C. C. & Hollstein, M. Clinical implications of the p53 tumor-suppressor gene. N. Engl. J. Med. 329, 1318–1327 (1993).
Moll, U. M., Riou, G. & Levine, A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc. Natl Acad. Sci. USA 89, 7262–7266 (1992).
Horak, E. et al. Mutant p53, EGF receptor and c-erbB-2 expression in human breast cancer. Oncogene 6, 2277–2284 (1991).
Moll, U. M., LaQuaglia, M., Benard, J. & Riou, G. Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc. Natl Acad. Sci. USA 92, 4407–4411 (1995).
Isaacs, J. S., Hardman, R., Carman, T. A., Barrett, J. C. & Weissman, B. E. Differential subcellular p53 localization and function in N- and S-type neuroblastoma cell lines. Cell Growth Differ. 9, 545–555 (1998).
Flamini, G. et al. Prognostic significance of cytoplasmic p53 overexpression in colorectal cancer. An immunohistochemical analysis. Eur. J. Cancer 32A, 802–806 (1996).
Runnebaum, I. B., Kieback, D. G., Mobus, V. J., Tong, X. W. & Kreienberg, R. Subcellular localization of accumulated p53 in ovarian cancer cells. Gynecol. Oncol. 61, 266–271 (1996).
Schlamp, C. L., Poulsen, G. L., Nork, T. M. & Nickells, R. W. Nuclear exclusion of wild-type p53 in immortalized human retinoblastoma cells. J. Natl Cancer Inst. 89, 1530–1536 (1997).
Moll, U. M. et al. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol. Cell. Biol. 16, 1126–1137 (1996).
Lu, W. et al. Nuclear exclusion of p53 in a subset of tumors requires MDM2 function. Oncogene 19, 232–240 (2000).
Sengupta, S., Vonesch, J. L., Waltzinger, C., Zheng, H. & Wasylyk, B. Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells. EMBO J. 19, 6051–6064 (2000).
Nikolaev, A. Y., Li, M., Puskas, N., Qin, J. & Gu, W. Parc: a cytoplasmic anchor for p53. Cell 112, 29–40 (2003). Evidence showing PARC is a cytoplasmic anchor protein and is important in p53 mislocalization by binding and sequestering p53 in the cytoplasm.
Craig, E., Zhang, Z. K., Davies, K. P. & Kalpana, G. V. A masked NES in INI1/hSNF5 mediates hCRM1-dependent nuclear export: implications for tumorigenesis. EMBO J. 21, 31–42 (2002). First report of an NES masking mechanism for INI1 export.
Versteege, I. et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394, 203–206 (1998).
Sevenet, N. et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum. Mol. Genet. 8, 2359–2368 (1999).
Biegel, J. A. et al. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin. Cancer Res. 8, 3461–3467 (2002).
Yung, E. et al. Inhibition of HIV-1 virion production by a transdominant mutant of integrase interactor 1. Nature Med. 7, 920–926 (2001).
Ae, K. et al. Chromatin remodeling factor encoded by ini1 induces G1 arrest and apoptosis in ini1-deficient cells. Oncogene 21, 3112–3120 (2002).
Versteege, I., Medjkane, S., Rouillard, D. & Delattre, O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene 21, 6403–6412 (2002).
Betz, B. L., Strobeck, M. W., Reisman, D. N., Knudsen, E. S. & Weissman, B. E. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene 21, 5193–5203 (2002).
Zhang, Z. K. et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol. Cell. Biol. 22, 5975–5988 (2002).
Giles, R. H., van Es, J. H. & Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653, 1–24 (2003).
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Henderson, B. R. & Fagotto, F. The ins and outs of APC and β-catenin nuclear transport. EMBO Rep. 3, 834–839 (2002).
Fabbro, M. & Henderson, B. R. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp. Cell Res. 282, 59–69 (2003).
Miyoshi, Y. et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum. Mol. Genet. 1, 229–233 (1992).
Powell, S. M. et al. APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–237 (1992).
Nagase, H. & Nakamura, Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum. Mutat. 2, 425–434 (1993).
Rosin-Arbesfeld, R., Cliffe, A., Brabletz, T. & Bienz, M. Nuclear export of the APC tumour suppressor controls β-catenin function in transcription. EMBO. J. 22, 1101–1113 (2003). Indicates that APC truncations might contribute to β-catenin nuclear localization and oncogenesis.
Yokoya, F., Imamoto, N., Tachibana, T. & Yoneda, Y. β-catenin can be transported into the nucleus in a Ran-unassisted manner. Mol. Biol. Cell 10, 1119–1131 (1999).
Fagotto, F., Gluck, U. & Gumbiner, B. M. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 8, 181–190 (1998).
Malik, H. S., Eickbush, T. H. & Goldfarb, D. S. Evolutionary specialization of the nuclear targeting apparatus. Proc. Natl Acad. Sci. USA 94, 13738–13742 (1997).
Suh, E. K. & Gumbiner, B. M. Translocation of β-catenin into the nucleus independent of interactions with FG-rich nucleoporins. Exp. Cell Res. 290, 447–456 (2003).
Neufeld, K. L. et al. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc. Natl Acad. Sci. USA 97, 12085–12090 (2000).
Henderson, B. R. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nature Cell Biol. 2, 653–660 (2000).
Neufeld, K. L., Zhang, F., Cullen, B. R. & White, R. L. APC-mediated downregulation of β-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep. 1, 519–523 (2000).
Rosin-Arbesfeld, R., Townsley, F. & Bienz, M. The APC tumour suppressor has a nuclear export function. Nature 406, 1009–1012 (2000).
Eleftheriou, A., Yoshida, M. & Henderson, B. R. Nuclear export of human β-catenin can occur independent of CRM1 and the adenomatous polyposis coli tumor suppressor. J. Biol. Chem. 276, 25883–25888 (2001).
Kim, I. S. et al. Truncated form of importin α identified in breast cancer cell inhibits nuclear import of p53. J. Biol. Chem. 275, 23139–23145 (2000). Truncated form of karyopherin-α in breast cancer cells leads to p53 cytoplasmic mislocalization and might contribute to tumorigenesis.
Brinkmann, U., Gallo, M., Polymeropoulos, M. H. & Pastan, I. The human CAS (cellular apoptosis susceptibility) gene mapping on chromosome 20q13 is amplified in BT474 breast cancer cells and part of aberrant chromosomes in breast and colon cancer cell lines. Genome Res. 6, 187–194 (1996).
Behrens, P., Brinkmann, U., Fogt, F., Wernert, N. & Wellmann, A. Implication of the proliferation and apoptosis associated CSE1L/CAS gene for breast cancer development. Anticancer Res. 21, 2413–2417 (2001).
Wellmann, A. et al. High expression of the proliferation and apoptosis associated CSE1L/CAS gene in hepatitis and liver neoplasms: correlation with tumor progression. Int. J. Mol. Med. 7, 489–494 (2001).
Brinkmann, U., Brinkmann, E., Gallo, M. & Pastan, I. Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc. Natl Acad. Sci. USA 92, 10427–10431 (1995).
Kutay, U., Bischoff, F. R., Kostka, S., Kraft, R. & Gorlich, D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell 90, 1061–1071 (1997).
Brinkmann, U., Brinkmann, E., Gallo, M., Scherf, U. & Pastan, I. Role of CAS, a human homologue to the yeast chromosome segregation gene CSE1, in toxin and tumor necrosis factor mediated apoptosis. Biochemistry 35, 6891–6899 (1996).
Ogryzko, V. V., Brinkmann, E., Howard, B. H., Pastan, I. & Brinkmann, U. Antisense inhibition of CAS, the human homologue of the yeast chromosome segregation gene CSE1, interferes with mitosis in HeLa cells. Biochemistry 36, 9493–9500 (1997).
Scherf, U., Pastan, I., Willingham, M. C. & Brinkmann, U. The human CAS protein which is homologous to the CSE1 yeast chromosome segregation gene product is associated with microtubules and mitotic spindle. Proc. Natl Acad. Sci. USA 93, 2670–2674 (1996).
Behrens, P., Brinkmann, U. & Wellmann, A. CSE1L/CAS: its role in proliferation and apoptosis. Apoptosis 8, 39–44 (2003).
Scherf, U., Kalab, P., Dasso, M., Pastan, I. & Brinkmann, U. The hCSE1/CAS protein is phosphorylated by HeLa extracts and MEK-1: MEK-1 phosphorylation may modulate the intracellular localization of CAS. Biochem. Biophys. Res. Commun. 250, 623–628 (1998).
Tomiyasu, T., Sasaki, M., Kondo, K. & Okada, M. Chromosome banding studies in 106 cases of chronic myelogenous leukemia. Jinrui Idengaku Zasshi 27, 243–258 (1982).
Borrow, J. et al. The t(7;11)(p15;p15) translocation in acute myeloid leukaemia fuses the genes for nucleoporin NUP98 and class I homeoprotein HOXA9. Nature Genet. 12, 159–167 (1996).
Nakamura, T. et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nature Genet. 12, 154–158 (1996). References 110 and 111 are the first reports to describe the NUP98–HOXA9 fusion in AML.
Fornerod, M. et al. Relocation of the carboxy terminal part of CAN from the nuclear envelope to the nucleus as a result of leukemia-specific chromosome rearrangements. Oncogene 10, 1739–1748 (1995).
Krumlauf, R. Hox genes in vertebrate development. Cell 78, 191–201 (1994).
Lam, D. H. & Aplan, P. D. NUP98 gene fusions in hematologic malignancies. Leukemia 15, 1689–1695 (2001).
Kasper, L. H. et al. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98–HOXA9 oncogenicity. Mol. Cell Biol. 19, 764–776 (1999). Demonstrated that the NUP98–HOXA9 fusion protein has transcriptional activity with p300 and CBP, explaining a possible mode of oncogenicity in AML.
Radu, A., Moore, M. S. & Blobel, G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell 81, 215–222 (1995).
Kudo, N. et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540–547 (1998).
Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051–1060 (1997).
Fukuda, M. et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308–311 (1997).
Ossareh-Nazari, B., Bachelerie, F. & Dargemont, C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278, 141–144 (1997).
Stade, K., Ford, C. S., Guthrie, C. & Weis, K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90, 1041–1050 (1997). References 118–121 identify CRM1 as the transport receptor for NES-containing cargo proteins.
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA 96, 9112–9117 (1999). Demonstrated that LMB covalently modifies CRM1, possibly through a Michael-type addition.
Akakura, S., Yoshida, M., Yoneda, Y. & Horinouchi, S. A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135). J. Biol. Chem. 276, 14649–14657 (2001).
Neville, M. & Rosbash, M. The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae. EMBO J. 18, 3746–3756 (1999).
Newlands, E. S., Rustin, G. J. & Brampton, M. H. Phase I trial of elactocin. Br. J. Cancer 74, 648–649 (1996).
Kau, T. R. et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN deficient tumor cells. Cancer Cell 4, 463–476 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
childhood acute lymphoblastic leukaemia
LocusLink
FURTHER INFORMATION
Advances in Cell Nucleus Research
Institute of Chemistry and Cell Biology and Initiative for Chemical Genetics
Glossary
- NUCLEAR-PORE COMPLEX
-
An aqueous channel that is composed of proteins (called nucleoporins) in the nuclear envelope, through which macromolecules move between the nucleus and cytoplasm.
- FORKHEAD TRANSCRIPTION-FACTOR SUPERFAMILY
-
A large superfamily of transcription factors, of which one family, FOXO, is phosphorylated and inhibited by AKT/PKB.
- COWDEN'S DISEASE
-
Also known as multiple harmatoma syndrome. An autosomal-dominant condition that is marked by hereditary predisposition for developing cancers due to mutation of the PTEN gene.
- LEPTOMYCIN B
-
A natural product that is isolated from Streptomyces and that inhibits CRM1 export activity by covalently binding to the Cys528 residue and preventing CRM1–substrate binding.
Rights and permissions
About this article
Cite this article
Kau, T., Way, J. & Silver, P. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 4, 106–117 (2004). https://doi.org/10.1038/nrc1274
Issue Date:
DOI: https://doi.org/10.1038/nrc1274
This article is cited by
-
Nuclear transport maintenance of USP22-AR by Importin-7 promotes breast cancer progression
Cell Death Discovery (2023)
-
The efficacy of selinexor (KPT-330), an XPO1 inhibitor, on non-hematologic cancers: a comprehensive review
Journal of Cancer Research and Clinical Oncology (2023)
-
The importin beta superfamily member RanBP17 exhibits a role in cell proliferation and is associated with improved survival of patients with HPV+ HNSCC
BMC Cancer (2022)
-
The interaction between S100A2 and KPNA2 mediates NFYA nuclear import and is a novel therapeutic target for colorectal cancer metastasis
Oncogene (2022)
-
Inferring differential subcellular localisation in comparative spatial proteomics using BANDLE
Nature Communications (2022)