Abstract
The ability to tag a protein at its endogenous locus with a fluorescent protein (FP) enables quantitative understanding of protein dynamics at the physiological level. Genome-editing technology has now made this powerful approach routinely applicable to mammalian cells and many other model systems, thereby opening up the possibility to systematically and quantitatively map the cellular proteome in four dimensions. 3D time-lapse confocal microscopy (4D imaging) is an essential tool for investigating spatial and temporal protein dynamics; however, it lacks the required quantitative power to make the kind of absolute and comparable measurements required for systems analysis. In contrast, fluorescence correlation spectroscopy (FCS) provides quantitative proteomic and biophysical parameters such as protein concentration, hydrodynamic radius, and oligomerization but lacks the capability for high-throughput application in 4D spatial and temporal imaging. Here we present an automated experimental and computational workflow that integrates both methods and delivers quantitative 4D imaging data in high throughput. These data are processed to yield a calibration curve relating the fluorescence intensities (FIs) of image voxels to the absolute protein abundance. The calibration curve allows the conversion of the arbitrary FIs to protein amounts for all voxels of 4D imaging stacks. Using our workflow, users can acquire and analyze hundreds of FCS-calibrated image series to map their proteins of interest in four dimensions. Compared with other protocols, the current protocol does not require additional calibration standards and provides an automated acquisition pipeline for FCS and imaging data. The protocol can be completed in 1 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cong, L., Ran, F., Cox, D., Lin, S. & Barretto, R. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Shen, B. et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Trevino, A.E. & Zhang, F. in Methods in Enzymology 546, 161–174 (Elsevier, 2014).
Koch, B. et al. Generation and validation of homozygous fluorescent knock-in cells using CRISPR–Cas9 genome editing. Nat. Protoc. http://dx.doi.org/10.1038/nprot.2018.042 (2018).
Digman, M.A., Stakic, M. & Gratton, E. in Methods in Enzymology 518, 121–144 (Elsevier, 2013).
Krieger, J.W. et al. Imaging fluorescence (cross-) correlation spectroscopy in live cells and organisms. Nat. Protoc. 10, 1948–74 (2015).
Bacia, K., Kim, S.A. & Schwille, P. Fluorescence cross-correlation spectroscopy in living cells. Nat. Methods 3, 83–89 (2006).
Bacia, K. & Schwille, P. Practical guidelines for dual-color fluorescence cross-correlation spectroscopy. Nat. Protoc. 2, 2842–2856 (2007).
Elson, E.L. Fluorescence correlation spectroscopy: past, present, future. Biophys. J. 101, 2855–2870 (2011).
Digman, M.A. & Gratton, E. Lessons in fluctuation correlation spectroscopy. Annu. Rev. Phys. Chem. 62, 645–668 (2011).
Mahen, R. et al. Comparative assessment of fluorescent transgene methods for quantitative imaging in human cells. Mol. Biol. Cell 25, 3610–3618 (2014).
Walther, N. et al. A quantitative map of human condensins provides new insights into mitotic chromosome architecture. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201801048 (2018).
Cuylen, S. et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 535, 308–312 (2016).
Germier, T. et al. Real-time imaging of a single gene reveals transcription-initiated local confinement. Biophys. J. 113, 1383–1394 (2017).
Cai, Y. et al. An experimental and computational framework to build a dynamic protein atlas of human cell division. bioRxiv http://dx.doi.org/10.1101/227751 (2017).
Conrad, C. et al. Micropilot: automation of fluorescence microscopy-based imaging for systems biology. Nat. Methods 8, 246–249 (2011).
Tischer, C., Hilsenstein, V., Hanson, K. & Pepperkok, R. Adaptive fluorescence microscopy by online feedback image analysis. Methods Cell Biol. 123, 489–503 (2014).
Wachsmuth, M. et al. High-throughput fluorescence correlation spectroscopy enables analysis of proteome dynamics in living cells. Nat. Biotechnol. 33, 384–389 (2015).
Kremers, G.-J., Gilbert, S.G., Cranfill, P.J., Davidson, M.W. & Piston, D.W. Fluorescent proteins at a glance. J. Cell Sci. 124, 157–160 (2011).
Shaner, N.C. et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 10, 407–409 (2013).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Bindels, D.S. et al. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56 (2016).
Ori, A. et al. Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Mol. Syst. Biol. 9, 648 (2013).
Suzuki, A., Badger, B.L. & Salmon, E.D. A quantitative description of Ndc80 complex linkage to human kinetochores. Nat. Commun. 6, 1–14 (2015).
Weir, J.R. et al. Insights from biochemical reconstitution into the architecture of human kinetochores. Nature 537, 249–253 (2016).
Bauer, M., Cubizolles, F., Schmidt, A. & Nigg, E.A. Quantitative analysis of human centrosome architecture by targeted proteomics and fluorescence imaging. EMBO J. 35, 1–15 (2016).
Beck, M. et al. The quantitative proteome of a human cell line. Mol. Syst. Biol. 7, 549 (2011).
Zacharias, D.A., Violin, J.D., Newton, A.C. & Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Cranfill, P.J. et al. Quantitative assessment of fluorescent proteins. Nat. Methods 13, 557–562 (2016).
Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 185, 1135–1148 (2009).
Bancaud, A., Huet, S., Rabut, G. & Ellenberg, J. Fluorescence perturbation techniques to study mobility and molecular dynamics of proteins in live cells: FRAP, photoactivation, photoconversion, and FLIP. Cold Spring Harb. Protoc. 2010, pdb.top90 (2010).
Merzlyak, E.M. et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods 4, 555–557 (2007).
Lam, A.J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012).
Khmelinskii, A. et al. Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat. Biotechnol. 30, 708–714 (2012).
Schwanhüusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Wu, J.-Q., McCormick, C.D. & Pollard, T.D. in Methods in Cell Biology 89, 253–273 (Elsevier, 2008).
Verdaasdonk, J.S., Lawrimore, J. & Bloom, K. in Methods in Cell Biology 123, 347–365 (Elsevier, 2014).
Wu, J.-Q. & Pollard, T.D. Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310–314 (2005).
Joglekar, A.P., Bouck, D.C., Molk, J.N., Bloom, K.S. & Salmon, E.D. Molecular architecture of a kinetochore-microtubule attachment site. Nat. Cell Biol. 8, 581–585 (2006).
Weidemann, T. et al. Counting nucleosomes in living cells with a combination of fluorescence correlation spectroscopy and confocal imaging. J. Mol. Biol. 334, 229–240 (2003).
Maeder, C.I. et al. Spatial regulation of Fus3 MAP kinase activity through a reaction-diffusion mechanism in yeast pheromone signalling. Nat. Cell Biol. 9, 1319–1326 (2007).
Shivaraju, M. et al. Cell-cycle-coupled structural oscillation of centromeric nucleosomes in yeast. Cell 150, 304–316 (2012).
Ulbrich, M.H. & Isacoff, E.Y. Subunit counting in membrane-bound proteins. Nat. Methods 4, 319 (2007).
Ulbrich, M.H. in Springer Series on Fluorescence 13, 263–291 (2011).
Ta, H., Wolfrum, J. & Herten, D.-P. An extended scheme for counting fluorescent molecules by photon-antibunching. Laser Phys. 20, 119–124 (2010).
Ta, H. et al. Mapping molecules in scanning far-field fluorescence nanoscopy. Nat. Commun. 6, 7977 (2015).
Lawrimore, J., Bloom, K.S. & Salmon, E.D. Point centromeres contain more than a single centromere-specific Cse4 (CENP-A) nucleosome. J. Cell Biol. 195, 573–582 (2011).
Charpilienne, A. et al. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J. Biol. Chem. 276, 29361–29367 (2001).
Picco, A., Mund, M., Ries, J., Nédélec, F. & Kaksonen, M. Visualizing the functional architecture of the endocytic machinery. Elife 4, e04535 (2015).
Keppler, A. et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 21, 86–89 (2003).
Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128–136 (2008).
Los, G.V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Mütze, J., Ohrt, T. & Schwille, P. Fluorescence correlation spectroscopy in vivo. Laser Photonics Rev. 5, 52–67 (2011).
Lukinavicius, G. et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy. Nat. Commun. 6, 8497 (2015).
Neumann, B. et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464, 721–727 (2010).
Otsuka, S. et al. Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope. Elife 5, e19071 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Schindelin, J., Rueden, C.T., Hiner, M.C. & Eliceiri, K.W. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529 (2015).
Rüttinger Buschmann, V., Krämer, B., Erdmann, R., MacDonald, R. & Koberling, F.S. et al. Comparison and accuracy of methods to determine the confocal volume for quantitative fluorescence correlation spectroscopy. J. Microsc. 232, 343–352 (2008).
Capoulade, J., Wachsmuth, M., Hufnagel, L. & Knop, M. Quantitative fluorescence imaging of protein diffusion and interaction in living cells. Nat. Biotechnol. 29, 835–839 (2011).
Kapusta, P. Absolute Diffusion Coefficients: Compilation of Reference Data for FCS Calibration https://www.picoquant.com/images/uploads/page/files/7353/appnote_diffusioncoefficients.pdf (2010).
Acknowledgements
We thank the mechanical and electronics workshops of EMBL for custom hardware, the Advanced Light Microscopy Facility of EMBL for microscopy support, and the Flow Cytometry Core Facility of EMBL for cell sorting. We gratefully acknowledge B. Nijmeijer, S. Alexander, and A. Rybina for critical reading of the manuscript. We thank T. Ohrt (Zeiss) for support in the hardware control. This work was supported by grants to J.E. from the European Commission EU-FP7-Systems Microscopy NoE (grant agreement 258068), EU-FP7-MitoSys (grant agreement 241548), and iNEXT (grant agreement 653706), as well as by EMBL (A.Z.P., Y.C., N.W., M.J.H., B.K., M.W., and J.E.). Y.C. and N.W. were supported by the EMBL International PhD Programme (EIPP).
Author information
Authors and Affiliations
Contributions
A.Z.P. performed the experiments and analysis, and designed the software packages. A.Z.P. developed the protocol with the help of Y.C. and M.W. N.W. tested the protocol and helped in writing the software manuals. B.K. created the homozygous cell line and provided the dextran-labeling protocol. M.J.H. provided the code to segment the cells. A.Z.P. wrote the protocol with the help of N.W., B.K., and J.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
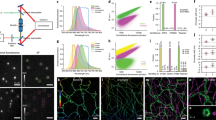
Supplementary Figure 1 Quality control of FCS traces
(a) Typical trace that passes the quality control (QC) according to the parameters shown in f. The QC is based on thresholds applied to fitting parameters such as the sum of squared residuals χ2, the coefficient of variation R2, and properties of the photon counts traces (e.g. Bleach coefficient, as calculated by FA). A visual inspection of the photon counts and autocorrelation traces can also be used for quality control. Scale bar 10 μm. (b) FCS measurement point is at the boundary of the cytoplasmic compartment causing a decreasing drift in photon counts. (c) FCS measurement point is at the boundary of the cytoplasmic compartment causing an increasing drift in photon counts. (d) FCS measurement point is at the boundary between a dim and a brighter cell causing large fluctuations in the photon counts. (e) FCS measurement point is in a cell that does not express a fluorescent protein. (f) Table of parameters used for the QC. The measurements in b and c do not pass the QC according to the bleach parameter. The measurement in d does not pass the QC according to the χ2 value. The measurement in e does not pass the QC according to the R2 value. None of the traces in b-e pass the visual inspection. The thresholds used were χ2 < 1.2, R2 > 0.9, 0.8 < Bleach < 1.2. The thresholds can be interactively set in the FCSCalibration software (Supplementary Software 4).
Supplementary Figure 2 CPM and effective volume dependency on pinhole diameter and laser power
(a) Counts per molecule (CPM) as function of the pinhole diameter for Alexa488 in water. (b) Effective confocal focal volume estimated as described in the supplement (Supplementary Notes 1–2 and Box 2) as function of the pinhole diameter for Alexa488. (c) Effective confocal focal volume as function of the excitation laser (Argon 488 nm). The pinhole was 34 μm.
Supplementary Figure 3 Simulated FCS-calibrated imaging
(a) Illustration of a homogeneous fluorophore distribution. (b) The slope of the fluorophore concentration as function of pixel fluorescence intensity gives the calibration coefficient. Simulated PSF is a 3D Gaussian (Eq. S1). The size of the PSF is characterized by its e2 decay w0 and z0 in XY and Z direction, respectively. Here and in all subsequent panels background and detector noise are not simulated. (c) Simulated Z-stack of a point source (50 fluorophores). (d) Using the calibration coefficient and Eqs. S15, S16, and S19 the total number of proteins in each plane is calculated. Several planes along the Z-direction are summed (circles). The expected protein number is obtained when a large region along Z is considered (>2*z0). Simulated images have a pixel-size of 0.4, 0.6, 0.8, 1 times w0 in all directions (different colors). Triangles give the result when all pixels are considered (Eq. S19). Squares give the approximation using the integral of the PSF along Z (Eq. S21). Diamonds give the approximation when the integral of the whole PSF and the pixel with the highest intensity is used (Eq. S23). (e) Simulated fluorophores distributed in a XZ plane (density of 100 fluorophores/μm2). Scale bar 10 μm. (f) Protein density is computed using Eqs. S15 and S24. The expected density is obtained for a region width along the X direction of > 2*w0. Triangles give the result when all pixels are considered. Squares give the approximation when the integral of the PSF along X is used (Eq. S26). (g) Simulated fluorophores distributed in a XY plane (density of 100 fluorophores/μm2). (h) The average protein density is summed for planes along Z (Eqs. S15 and S17). The expected density is obtained for a region width > 2*z0. Triangles give the result when all pixels are considered. Squares give the approximation when the integral of the PSF along Z is used (Eq. S29). Each fluorophore has a simulated intensity of 1000 (a.u.), the simulated PSF is characterized by w0 = 250 nm, z0 = 1500 nm. The black lines give the theoretically expected result after integration of the Gaussian function  where N e is the expected protein number/density (50 in d, and 100 in f and h) and Rs is the region size in unit of the PSF characteristic size.
where N e is the expected protein number/density (50 in d, and 100 in f and h) and Rs is the region size in unit of the PSF characteristic size.
Supplementary information
Supplementary Text and Figures
Supplementary figures 1–3, Supplementary Tables 1–2 and Supplementary Notes 1–6 (PDF 1377 kb)
Supplementary Software 1
The FCSRunner software to manually acquire FCS and imaging data. (ZIP 2057 kb)
Supplementary Software 2
The MyPiC software to automatically acquire FCS and imaging data using adaptive-feedback microscopy. (ZIP 7646 kb)
Supplementary Software 3
A FiJi software package to automatically acquire FCS and imaging data using adaptive-feedback microscopy; to be used in combination with Supplementary Software 2. (ZIP 7971 kb)
Supplementary Software 4
FiJi and R tools to analyze the FCS and imaging data and compute image calibration coefficients. (ZIP 114307 kb)
Rights and permissions
About this article
Cite this article
Politi, A., Cai, Y., Walther, N. et al. Quantitative mapping of fluorescently tagged cellular proteins using FCS-calibrated four-dimensional imaging. Nat Protoc 13, 1445–1464 (2018). https://doi.org/10.1038/nprot.2018.040
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.040
This article is cited by
-
Asymmetric oligomerization state and sequence patterning can tune multiphase condensate miscibility
Nature Chemistry (2024)
-
Genetically encoded multimeric tags for subcellular protein localization in cryo-EM
Nature Methods (2023)
-
RNA polymerase II depletion from the inactive X chromosome territory is not mediated by physical compartmentalization
Nature Structural & Molecular Biology (2023)
-
A quantitative map of nuclear pore assembly reveals two distinct mechanisms
Nature (2023)
-
Single-cell quantification and dose-response of cytosolic siRNA delivery
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.