Abstract
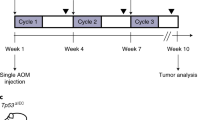
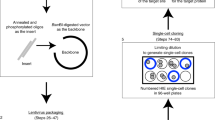
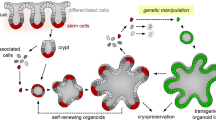
Most genetically engineered mouse models (GEMMs) of colorectal cancer are limited by tumor formation in the small intestine, a high tumor burden that limits metastasis, and the need to generate and cross mutant mice. Cell line or organoid transplantation models generally produce tumors in ectopic locations—such as the subcutaneous space, kidney capsule, or cecal wall—that do not reflect the native stromal environment of the colon mucosa. Here, we describe detailed protocols to rapidly and efficiently induce site-directed tumors in the distal colon of mice that are based on colonoscopy-guided mucosal injection. These techniques can be adapted to deliver viral vectors carrying Cre recombinase, CRISPR–Cas9 components, CRISPR-engineered mouse tumor organoids, or human cancer organoids to mice to model the adenoma–carcinoma–metastasis sequence of tumor progression. The colonoscopy injection procedure takes ∼15 min, including preparation. In our experience, anyone with reasonable hand–eye coordination can become proficient with mouse colonoscopy and mucosal injection with a few hours of practice. These approaches are ideal for a wide range of applications, including assessment of gene function in tumorigenesis, examination of tumor–stroma interactions, studies of cancer metastasis, and translational research with patient-derived cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel, R.L. et al. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 67, 177–193 (2017).
Roper, J. & Hung, K.E. Priceless GEMMs: genetically engineered mouse models for colorectal cancer drug development. Trends Pharmacol. Sci. 33, 449–455 (2012).
Roper, J., Martin, E.S. & Hung, K.E. Overview of genetically engineered mouse models of colorectal carcinoma to enable translational biology and drug development. Curr. Protoc. Pharmacol. 65, 14.29.1–14.29.10 (2014).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Su, L.K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Hung, K.E. et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc. Natl. Acad. Sci. USA 107, 1565–1570 (2010).
Hadac, J.N. et al. Colon tumors with the simultaneous induction of driver mutations in APC, KRAS, and PIK3CA still progress through the adenoma-to-carcinoma sequence. Cancer Prev. Res. (Phila.) 8, 952–961 (2015).
Golovko, D., Kedrin, D., Yilmaz, Ö.H. & Roper, J. Colorectal cancer models for novel drug discovery. Expert Opin. Drug Discov. 10, 1217–1229 (2015).
Melo, F. & de, S.E. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
O'Rourke, K.P. et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35, 577–582 (2017).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Fumagalli, A. et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl. Acad. Sci. USA 114, E2357–E2364 (2017).
Fumagalli, A. et al. A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression. Nat. Protoc. http://dx.doi.org/10.1038/nprot.2017.137.
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Enquist, I.B. et al. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat. Commun. 5, 3530 (2014).
Xue, X. et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 24, 447–461 (2016).
Huang, E.H. et al. Colonoscopy in mice. Surg. Endosc. 16, 22–24 (2002).
Wirtz, S., Becker, C., Blumberg, R., Galle, P.R. & Neurath, M.F. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J. Immunol. 168, 411–420 (2002).
Funovics, M.A. et al. Miniaturized multichannel near infrared endoscope for mouse imaging. Mol. Imaging 2, 350–357 (2003).
Hensley, H., Cooper, H.S., Chang, W.-C.L. & Clapper, M.L. Imaging matrix metalloproteases in spontaneous colon tumors: validation by correlation with histopathology. Methods Mol. Biol. 1579, 245–255 (2017).
Rabinsky, E.F. et al. Overexpressed claudin-1 can be visualized endoscopically in colonic adenomas in vivo. Cell. Mol. Gastroenterol. Hepatol. 2, 222–237 (2016).
Gounaris, E., Ishihara, Y., Shrivastrav, M., Bentrem, D. & Barrett, T.A. Near-Infrared fluorescence endoscopy to detect dysplastic lesions in the mouse colon. Methods Mol. Biol. 1422, 137–147 (2016).
Tumlinson, A.R. et al. Endoscope-tip interferometer for ultrahigh. Opt. Express 14, 1878–1887 (2006).
Hariri, L.P. et al. Endoscopic optical coherence tomography and laser-induced fluorescence spectroscopy in a murine colon cancer model. Lasers Surg. Med. 38, 305–313 (2006).
Becker, C. et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 54, 950–954 (2005).
Miller, S.J. et al. In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PLoS One 6, e17384 (2011).
Mitsunaga, M. et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut 62, 1179–1186 (2013).
Waldner, M.J., Wirtz, S., Neufert, C., Becker, C. & Neurath, M.F. Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat. Protoc. 6, 1471–1481 (2011).
Becker, C., Fantini, M.C. & Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 1, 2900–2904 (2007).
Roper, J. et al. Combination PI3K/MEK inhibition promotes tumor apoptosis and regression in PIK3CA wild-type, KRAS mutant colorectal cancer. Cancer Lett. 347, 204–211 (2014).
Zigmond, E. et al. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One 6, e28858 (2011).
Bettenworth, D. et al. Endoscopy-guided orthotopic implantation of colorectal cancer cells results in metastatic colorectal cancer in mice. Clin. Exp. Metastasis 33, 551–562 (2016).
Roper, J. et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 35, 569–576 (2017).
Sánchez-Rivera, F.J. et al. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 516, 428–431 (2014).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Koo, B.-K., Sasselli, V. & Clevers, H. Retroviral gene expression control in primary organoid cultures. Curr. Protoc. Stem Cell Biol. 27, Unit 5A.6 (2013).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Platt, R.J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Kuraguchi, M. et al. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2, e146 (2006).
Akama-Garren, E.H. et al. A modular assembly platform for rapid generation of DNA constructs. Sci. Rep. 6, 16836 (2016).
Miyoshi, H. & Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Tammela, T. et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 545, 355–359 (2017).
DuPage, M. et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19, 72–85 (2011).
DuPage, M., Dooley, A.L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Roper, J. et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA wild-type colorectal cancer. PLoS One 6, e25132 (2011).
Druckrey, H. Production of colonic carcinomas by 1, 2-dialkylhydrazines and azoxyalkanes in Burdette, W. (ed) 267–279 (1970).
Tanaka, T. et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94, 965–973 (2003).
Neufert, C., Becker, C. & Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998–2004 (2007).
Boutin, A.T. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017).
Xue, Y., Johnson, R., Desmet, M., Snyder, P.W. & Fleet, J.C. Generation of a transgenic mouse for colorectal cancer research with intestinal cre expression limited to the large intestine. Mol. Cancer Res. 8, 1095–1104 (2010).
Tetteh, P.W. et al. Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research. Proc. Natl. Acad. Sci. USA 113, 11859–11864 (2016).
Hinoi, T. et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 67, 9721–9730 (2007).
Dow, L.E. et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161, 1539–1552 (2015).
Martin, E.S. et al. Development of a colon cancer GEMM-derived orthotopic transplant model for drug discovery and validation. Clin. Cancer Res. 19, 2929–2940 (2013).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) (K08 CA198002 to J.R.; K99 CA187317 to T.T.; and R00 AG045144 and R01 CA211184 to Ö.H.Y.), the Department of Defense (PRCRP Career Development Award CA120198 to J.R.), the V Foundation V Scholar Award (to J.R. and Ö.H.Y.), the Sidney Kimmel Scholar Award (to Ö.H.Y.), the Pew-Stewart Trust Scholar Award (to Ö.H.Y.), the Koch Institute Frontier Research Program through the Kathy and Curt Marble Cancer Research Fund (to Ö.H.Y.), the American Federation of Aging Research (AFAR; to Ö.H.Y.), and the Hope Funds for Cancer Research (to T.T.), as well as by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. We thank the Swanson Biotechnology Center at the Koch Institute for technical support, specifically K. Cormier and C. Condon at the Hope Babette Tang (1983) Histology Facility; and S. Holder for histology support. The research was conducted in compliance with the Animal Welfare Act Regulations and other federal statutes relating to animals and experiments involving animals, and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996. We thank M. Tschurtschenthaler, R.-F. Jackstadt, J. Leach, and P. Westcott for critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
J.R. designed and performed all experiments. T.T. assisted in design, construction, and production of lentiviral vectors. A.A., M.A., and S.B.S. assisted with mucosal injection experiments. T.J. participated in the interpretation of results. Ö.H.Y. supervised the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Demonstration of sample preparation and transfer to injection needle.
This procedure is performed by the assistant. The sample is drawn into the syringe using the transfer needle. Then the transfer needle is removed, and the syringe is attached to the injection needle. When the operator is ready, the assistant quickly injects the sample. (MP4 23729 kb)
Demonstration of colonoscopy-guided mucosal injection.
The operator places the tip of the injection needle into the mucosa of the colon. Then, the operator asks the assistant to rapidly inject the sample. The injection forms a mucosal 'bubble' by overcoming the resistance of the tissue. Appropriate institutional regulatory board permission was obtained for these experiments. (MP4 20621 kb)
White-light colonoscopy of a colon tumor generated by somatic CRISPR–Cas9 editing of the Apc tumor suppressor gene.
Tamoxifen-treated Rosa26LSL-Cas9-eGFP/+;VillinCreER mice were injected under colonoscopy guidance with U6::sgApc-EFS::turboRFP lentivirus (titer = 10,000 TU/μl). Tumors were visualized by colonoscopy ~6 weeks after viral injection. In this video, a large tumor is seen with white-light colonoscopy ~1 year after viral injection. Appropriate institutional regulatory board permission was obtained for these experiments. (MP4 4110 kb)
GFP fluorescence colonoscopy of a colon tumor generated by somatic CRISPR–Cas9 editing of the Apc tumor suppressor gene.
Tamoxifen-treated Rosa26LSL-Cas9-eGFP/+;VillinCreER mice were injected under colonoscopy guidance with U6::sgApc-EFS::turboRFP lentivirus (titer = 10,000 TU/μl). Tumors were visualized by colonoscopy ~6 weeks after viral injection. In this video, a large tumor is seen with GFP fluorescence colonoscopy ~1 year after viral injection. Appropriate institutional regulatory board permission was obtained for these experiments. (MP4 3805 kb)
RFP fluorescence colonoscopy of a colon tumor generated by somatic CRISPR–Cas9 editing of the Apc tumor suppressor gene.
Tamoxifen-treated Rosa26LSL-Cas9-eGFP/+;VillinCreER mice were injected under colonoscopy guidance with U6::sgApc-EFS::turboRFP lentivirus (titer = 10,000 TU/μl). Tumors were visualized by colonoscopy ~6 weeks after viral injection. In this video, a large tumor is seen with RFP fluorescence colonoscopy ~1 year after viral injection. Appropriate institutional regulatory board permission was obtained for these experiments. (MP4 5375 kb)
Inflammatory polyp formation following colonoscopy-guided mucosal injection.
If mucosal injection does not result in tumorigenesis, an inflammatory polyp occasionally forms at the injection site. These polyps can be distinguished from adenomatous polyps or tumors by colonoscopy features. Inflammatory polyps are thin and small, do not grow, and exhibit a vascular pattern that is similar to that of normal mucosa. Appropriate institutional regulatory board permission was obtained for these experiments. (MP4 6014 kb)
Rights and permissions
About this article
Cite this article
Roper, J., Tammela, T., Akkad, A. et al. Colonoscopy-based colorectal cancer modeling in mice with CRISPR–Cas9 genome editing and organoid transplantation. Nat Protoc 13, 217–234 (2018). https://doi.org/10.1038/nprot.2017.136
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.136
This article is cited by
-
Casein kinase 2 phosphorylates and induces the SALL2 tumor suppressor degradation in colon cancer cells
Cell Death & Disease (2024)
-
SOX17 enables immune evasion of early colorectal adenomas and cancers
Nature (2024)
-
The role of organoids in cancer research
Experimental Hematology & Oncology (2023)
-
Lentiviral in situ targeting of stem cells in unperturbed intestinal epithelium
BMC Biology (2023)
-
Mismatch repair deficiency is not sufficient to elicit tumor immunogenicity
Nature Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.