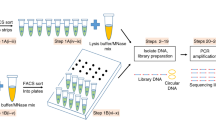
Abstract
Increased chromatin accessibility is a feature of cell-type-specific cis-regulatory elements; therefore, mapping of DNase I hypersensitive sites (DHSs) enables the detection of active regulatory elements of transcription, including promoters, enhancers, insulators and locus-control regions. Single-cell DNase sequencing (scDNase-seq) is a method of detecting genome-wide DHSs when starting with either single cells or <1,000 cells from primary cell sources. This technique enables genome-wide mapping of hypersensitive sites in a wide range of cell populations that cannot be analyzed using conventional DNase I sequencing because of the requirement for millions of starting cells. Fresh cells, formaldehyde-cross-linked cells or cells recovered from formalin-fixed paraffin-embedded (FFPE) tissue slides are suitable for scDNase-seq assays. To generate scDNase-seq libraries, cells are lysed and then digested with DNase I. Circular carrier plasmid DNA is included during subsequent DNA purification and library preparation steps to prevent loss of the small quantity of DHS DNA. Libraries are generated for high-throughput sequencing on the Illumina platform using standard methods. Preparation of scDNase-seq libraries requires only 2 d. The materials and molecular biology techniques described in this protocol should be accessible to any general molecular biology laboratory. Processing of high-throughput sequencing data requires basic bioinformatics skills and uses publicly available bioinformatics software.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Buenrostro, J.D., Giresi, P.G., Zaba, L.C., Chang, H.Y. & Greenleaf, W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Boyle, A.P. et al. High-resolution mapping and characterization of open chromatin across the genome. Cell 132, 311–322 (2008).
Crawford, G.E. et al. Genome-wide mapping of DNase hypersensitive sites using massively parallel signature sequencing (MPSS). Genome Res. 16, 123–131 (2006).
Crawford, G.E. et al. DNase-chip: a high-resolution method to identify DNase I hypersensitive sites using tiled microarrays. Nat. Methods 3, 503–509 (2006).
Jin, W. et al. Genome-wide detection of DNase I hypersensitive sites in single cells and FFPE tissue samples. Nature 528, 142–146 (2015).
Wen, L. & Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 17, 71 (2016).
Picelli, S. et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 24, 2033–2040 (2014).
Buenrostro, J.D. et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015).
Cusanovich, D.A. et al. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914 (2015).
Chen, X. et al. ATAC-see reveals the accessible genome by transposase-mediated imaging and sequencing. Nat. Methods 13, 1013–1020 (2016).
Song, L. et al. Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res. 21, 1757–1767 (2011).
Simon, J.M., Giresi, P.G., Davis, I.J. & Lieb, J.D. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat. Protoc. 7, 256–267 (2012).
Lu, F. et al. Establishing chromatin regulatory landscape during mouse preimplantation development. Cell 165, 1375–1388 (2016).
Zhang, X. et al. Single-cell sequencing for precise cancer research: progress and prospects. Cancer Res. 76, 1305–1312 (2016).
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
He, H.H. et al. Refined DNase-seq protocol and data analysis reveals intrinsic bias in transcription factor footprint identification. Nat. Methods 11, 73–78 (2014).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Robinson, J.T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Yue, F. et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364 (2014).
Acknowledgements
We thank W. Jin for his insights into the bioinformatics analysis of the data, the National Heart, Lung, and Blood Institute (NHLBI) DNA Sequencing Core facility for sequencing the libraries and the NHLBI Flow Cytometry Core facility for sorting the cells. The work was supported by the Division of Intramural Research, NHLBI (K.Z.).
Author information
Authors and Affiliations
Contributions
J.C. and K.Z. wrote the manuscript. J.C., Y.D., J.S. and K.Z planned and designed the experiments. J.C., Y.D. and K.Z. performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Library adaptor and multiplex PCR primer sequences. The table lists the DNA sequences for the library adaptors and PCR multiplexing primers compatible with the Illumina sequencing platform. The sequence information is derived from the Illumina Adapter Sequences Document (1000000002694 v01). When ordering the PCR primers, the final 3′ thymine should have a modified phosphorothioate bond (denoted by “*” in the table) to reduce exonuclease degradation. In addition, the 5′ end of the Illumina multiplexing adaptor-top should be phosphorylated (denoted by “P-” in the table). (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Cooper, J., Ding, Y., Song, J. et al. Genome-wide mapping of DNase I hypersensitive sites in rare cell populations using single-cell DNase sequencing. Nat Protoc 12, 2342–2354 (2017). https://doi.org/10.1038/nprot.2017.099
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.099
This article is cited by
-
Single-cell sequencing technology applied to epigenetics for the study of tumor heterogeneity
Clinical Epigenetics (2023)
-
Chromatin accessibility profiling methods
Nature Reviews Methods Primers (2021)
-
A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility
Nature Protocols (2021)
-
Genome-wide profiling of nucleosome position and chromatin accessibility in single cells using scMNase-seq
Nature Protocols (2020)
-
Genomic methods in profiling DNA accessibility and factor localization
Chromosome Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.