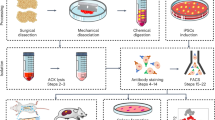
Abstract
Xenotransplantation is frequently used to study normal and malignant hematopoiesis of human cells. However, conventional mouse xenotransplantation models lack essential human-specific bone-marrow (BM)-microenvironment-derived survival, proliferation, and self-renewal signals for engraftment of normal and malignant blood cells. As a consequence, many human leukemias and other hematologic disorders do not robustly engraft in these conventional models. Here, we describe a complete workflow for the generation of humanized ossicles with an accessible BM microenvironment that faithfully recapitulates normal BM niche morphology and function. The ossicles, therefore, allow for accelerated and superior engraftment of primary patient-derived acute myeloid leukemia (AML) and other hematologic malignancies such as myelofibrosis (MF) in mice. The humanized ossicles are formed by in situ differentiation of BM-derived mesenchymal stromal cells (MSCs). Human hematopoietic cells can subsequently be transplanted directly into the ossicle marrow space or by intravenous injection. Using this method, a humanized engraftable BM microenvironment can be formed within 6–10 weeks. Engraftment of human hematopoietic cells can be evaluated by flow cytometry 8–16 weeks after transplantation. This protocol describes a robust and reproducible in vivo methodology for the study of normal and malignant human hematopoiesis in a more physiologic setting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bonnet, D. & Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Pearce, D.J. et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood 107, 1166–1173 (2006).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
McCune, J.M. et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241, 1632–1639 (1988).
Agliano, A. et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int. J. Cancer 123, 2222–2227 (2008).
Greiner, D.L., Hesselton, R.A. & Shultz, L.D. SCID mouse models of human stem cell engraftment. Stem Cells 16, 166–177 (1998).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106, 1565–1573 (2005).
Feuring-Buske, M. et al. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia 17, 760–763 (2003).
Goyama, S., Wunderlich, M. & Mulloy, J.C. Xenograft models for normal and malignant stem cells. Blood 125, 2630–2640 (2015).
Wunderlich, M. et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 24, 1785–1788 (2010).
Nicolini, F.E., Cashman, J.D., Hogge, D.E., Humphries, R.K. & Eaves, C.J. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia 18, 341–347 (2004).
Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 (2014).
Rongvaux, A. et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc. Natl. Acad. Sci. USA 108, 2378–2383 (2011).
Strowig, T. et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA 108, 13218–13223 (2011).
Willinger, T. et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc. Natl. Acad. Sci. USA 108, 2390–2395 (2011).
Sarry, J.E. et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J. Clin. Invest. 121, 384–395 (2011).
Sanchez, P.V. et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia 23, 2109–2117 (2009).
Patel, S. et al. Successful xenografts of AML3 samples in immunodeficient NOD/shi-SCID IL2Rgamma(−)/(−) mice. Leukemia 26, 2432–2435 (2012).
Kim, D., Park, C.Y., Medeiros, B.C. & Weissman, I.L. CD19-CD45 low/- CD38 high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 26, 2530–2537 (2012).
Kim, D. et al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 26, 2538–2545 (2012).
Pang, W.W. et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc. Natl. Acad. Sci. USA 110, 3011–3016 (2013).
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978).
Mendelson, A. & Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 20, 833–846 (2014).
Schepers, K., Campbell, T.B. & Passegue, E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell 16, 254–267 (2015).
Reinisch, A. et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat. Med. 22, 812–821 (2016).
Chao, M.P. et al. Human AML-iPSCs reacquire leukemic properties after differentiation and model clonal variation of disease Cell Stem Cell 26, 329–344 (2016).
Antonelli, A. et al. Establishing human leukemia xenograft mouse models by implanting human bone marrow-like scaffold-based niches. Blood 128, 2949–2959 (2016).
Abarrategi, A. et al. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J. Clin. Invest. 127, 543–548 (2017).
Janicki, P., Kasten, P., Kleinschmidt, K., Luginbuehl, R. & Richter, W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 6, 3292–3301 (2010).
Serafini, M. et al. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 12, 659–672 (2014).
Groen, R.W. et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood 120, e9–e16 (2012).
Holzapfel, B.M. et al. Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials 61, 103–114 (2015).
Martine, L.C. et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat. Protoc. 12, 639–663 (2017).
Scotti, C. et al. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 110, 3997–4002 (2013).
Scotti, C. et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 107, 7251–7256 (2010).
Scotti, C. et al. Engineering small-scale and scaffold-based bone organs via endochondral ossification using adult progenitor cells. Methods Mol. Biol. 1416, 413–424 (2016).
Pievani, A. et al. Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo. Development 144, 1035–1044 (2017).
Reinisch, A. et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 125, 249–260 (2015).
Chan, C.K. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Battula, V.L. et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood 122, 357–366 (2013).
Chen, Y. et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood 119, 4971–4980 (2012).
Sontakke, P. et al. Modeling BCR-ABL and MLL-AF9 leukemia in a human bone marrow-like scaffold-based xenograft model. Leukemia 30, 2064–2073 (2016).
Doucet, C. et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 205, 228–236 (2005).
Reinisch, A. et al. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen. Med. 2, 371–382 (2007).
Burnouf, T., Strunk, D., Koh, M.B. & Schallmoser, K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 76, 371–387 (2016).
Schallmoser, K. et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng. Part C Methods 14, 185–196 (2008).
Schallmoser, K. et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 47, 1436–1446 (2007).
Schallmoser, K. & Strunk, D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J. Vis. Exp. http://dx.doi.org/10.3791/1523 (2009).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Buhring, H.J. et al. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 1106, 262–271 (2007).
Notta, F., Doulatov, S. & Dick, J.E. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115, 3704–3707 (2010).
Miller, P.H. et al. Analysis of parameters that affect human hematopoietic cell outputs in mutant c-kit-immunodeficient mice. Exp. Hematol. 48, 41–49 (2017).
Song, J. et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood 115, 2592–2600 (2010).
Pettway, G.J. et al. Anabolic actions of PTH (1-34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone 36, 959–970 (2005).
Chevaleyre, J. et al. Busulfan administration flexibility increases the applicability of scid repopulating cell assay in NSG mouse model. PLoS One 8, e74361 (2013).
Kang, Y.K. et al. Humanizing NOD/SCID/IL-2Rgammanull (NSG) mice using busulfan and retro-orbital injection of umbilical cord blood-derived CD34(+) cells. Blood Res. 51, 31–36 (2016).
Saland, E. et al. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 5, e297 (2015).
Chhabra, A. et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci. Transl. Med. 8, 351ra105 (2016).
Czechowicz, A., Kraft, D., Weissman, I.L. & Bhattacharya, D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299 (2007).
Palchaudhuri, R. et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat. Biotechnol. 34, 738–745 (2016).
Taya, Y. et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science 354, 1152–1155 (2016).
McIntosh, B.E. et al. Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep. 4, 171–180 (2015).
Rahmig, S. et al. Improved human erythropoiesis and platelet formation in humanized NSGW41 mice. Stem Cell Rep. 7, 591–601 (2016).
Chung, Y.R., Kim, E. & Abdel-Wahab, O. Femoral bone marrow aspiration in live mice. J. Vis. Exp. 89, e51660 (2014).
Park, C.Y., Majeti, R. & Weissman, I.L. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat. Protoc. 3, 1932–1940 (2008).
Bartmann, C. et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion 47, 1426–1435 (2007).
Reinisch, A. et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood 113, 6716–6725 (2009).
Acknowledgements
We acknowledge the Tissue Bank of the Division of Hematology at Stanford University and the patients for donating their samples. We acknowledge F. Zhao for lab management, M. Stafford for technical help with ossicle generation, and D. Strunk for providing critical reagents, advice, and intellectual support for the initial development of the protocol. A.R. was supported by an Erwin-Schroedinger Research Fellowship (Austrian Science Fund). D.C.H. is a California Institute for Regenerative Medicine (CIRM) scholar. R.M. is a New York Stem Cell Foundation Robertson Investigator and Leukemia and Lymphoma Society Scholar. This research was supported by the Leukemia and Lymphoma Society, the New York Stem Cell Foundation, and National Institutes of Health grants R01CA188055 and U01HL099999 to R.M. This work was also supported by funding from the European Union's Horizon 2020 research and innovation programme under grant agreement number 668724 (TECHNOBEAT) to D. Strunk (Paracelsus Medical University of Salzburg, Austria) and grant agreement number 731377 (MUSIC) to K.S.
Author information
Authors and Affiliations
Contributions
A.R. and R.M. conceived and designed the project. A.R. and D.C.H. performed the experimental work. A.R. analyzed all data, K.S. provided critical reagents, and A.R. and R.M. wrote the protocol.
Corresponding authors
Ethics declarations
Competing interests
R.M. has an ownership interest (including patents) in Forty Seven Inc. and is a consultant/advisory board member for the same. The other authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Illustration of pHPL production.
(a) To prepare platelet concentrates from whole blood donations, four units of blood group (BG) O and one unit of BG AB whole blood donations should be separated into plasma, buffy coat (BF), and red blood cells by centrifugation. (b) One unit of platelet concentrate is generated by combining four buffy coat units, (all BG O) and one BG AB plasma. The combined product should be centrifuged again to separate leukocytes, which will thereafter be depleted through inline-filtration (as described in more detail in BOX1). These steps should be repeated until 10 units are collected. (c) Platelets within the concentrates are lysed by freezing and thawing, and the resulting human platelet lysates (HPL) are pooled to generate 2 – 3 liters of pooled HPL. After aliquoting into smaller storage bags, a second freeze/thaw cycle and one additional centrifugation step should be performed to guarantee maximal lysis and depletion of platelet fragments. The resulting product can be stored in 50mL tubes, ready to use for media preparation.
Supplementary Figure 2 Gating strategy to identify BM-MNCs and BM-MSCs.
(a) Flow-cytometric contour plot showing gating of mononuclear cells (MNCs, red box) within total nucleated cells (TNC, black box). Cell size (FSC-A, x-axis) and cellular granularity (SSC-A, y-axis) allow for the discrimination of lymphocytes (red), monocytes (green) and granulocytes (blue) as illustrated in (b). MNCs are comprised of lymphocytes and monocytes whereas TNCs additionally include granulocytes. (c) Left plot: Mesenchymal stromal cells (MSCs) are gated (black box) based on cell size (FSC-A, x-axis) and cellular granularity (SSC-A, y-axis) and can be clearly separate from contaminating debris (lower left). Right plot: Live cells (black box) are defined by absence of 7-aminoactinomycin D (7-AAD) staining. Arrow indicates that only cells defined by gate in FSC-A versus SSC-A plot are analyzed for 7-AAD.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2. (PDF 539 kb)
BM-MSC transplantation
Video demonstrating preparation of the mouse (shaving and skin disinfection) and subsequent injection of BM-MSCs admixed with extracellular matrix into subcutaneous mouse tissue at four different locations. After the procedure, the mouse is placed underneath a warm light source to guarantee quick recovery from anesthesia. (MP4 25576 kb)
Ossicle BM transplantation
Video showing direct intraossicle transplantation of human hematopoietic cells. (MP4 21890 kb)
Ossicle BM aspiration
Video demonstrating aspiration of hematopoietic cells directly from a humanized BM-ossicle niche. (MP4 23885 kb)
Rights and permissions
About this article
Cite this article
Reinisch, A., Hernandez, D., Schallmoser, K. et al. Generation and use of a humanized bone-marrow-ossicle niche for hematopoietic xenotransplantation into mice. Nat Protoc 12, 2169–2188 (2017). https://doi.org/10.1038/nprot.2017.088
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.088
This article is cited by
-
The evolution of preclinical models for myelodysplastic neoplasms
Leukemia (2024)
-
Gene therapy using haematopoietic stem and progenitor cells
Nature Reviews Genetics (2021)
-
TP53 mutated AML subclones exhibit engraftment in a humanized bone marrow ossicle mouse model
Annals of Hematology (2020)
-
Human multipotent hematopoietic progenitor cell expansion is neither supported in endothelial and endothelial/mesenchymal co-cultures nor in NSG mice
Scientific Reports (2019)
-
Humanized bone facilitates prostate cancer metastasis and recapitulates therapeutic effects of zoledronic acid in vivo
Bone Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.