Abstract
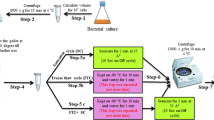
Fungal infections and their increasing resistance to antibiotics are an emerging threat to public health. Novel antifungal drugs, as well technologies that can help us bolster the antimicrobial pipeline and understand resistance mechanisms, are needed. The ergosterol biosynthetic pathway is one potential target for antifungal drugs. Here we describe how antifungal susceptibility testing can be combined with target identification in distal ergosterol biosynthesis by means of gas chromatography–mass spectrometry. The fungi are treated with sublethal doses of active components that block ergosterol biosynthesis, and the ergosterol biosynthesis intermediates are analyzed in a targeted metabolomics manner after derivatization (trimethylsilylation). Drug treatment results in distinct sterol patterns that are characteristic of the affected enzyme. Sterol identification based on relative retention times and electron ionization (EI) mass spectra, as well as semiquantitative assessment of ergosterol intermediates, is described. The protocol is applicable to yeasts and molds. The overall analysis time from incubation to test result is not more than 3 d. The assay can be used to determine whether an antifungal compound of interest targets sterol biosynthesis, and, if so, to determine which enzyme in the pathway it targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Denning, D.W. & Bromley, M.J. How to bolster the antifungal pipeline. Science 347, 1414–1416 (2015).
Brown, G.D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
The White House Briefing Room FACT SHEET: Obama Administration Releases National Action Plan to Combat Antibiotic-Resistant Bacteria. https://www.whitehouse.gov/the-press-office/2015/03/27/fact-sheet-obama-administration-releases-national-action-plan-combat-ant.
Centers for Disease Antibiotic Resistance Threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/.
Butler, M.S., Blaskovich, M.A. & Cooper, M.A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 66, 571–591 (2013).
Thompson Iii, G.R., Cadena, J. & Patterson, T.F. Overview of antifungal agents. Clin. Chest Med. 30, 203–215 (2009).
Miceli, M.H. & Kauffman, C.A. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 61, 1558–1565 (2015).
Petrikkos, G. & Skiada, A. Recent advances in antifungal chemotherapy. Int. J. Antimicrob. Agents 30, 108–117 (2007).
Kathiravan, M.K. et al. The biology and chemistry of antifungal agents: a review. Bioorg. Med. Chem. 20, 5678–5698 (2012).
Arendrup, M.C., Cuenca-Estrella, M., Lass-Flörl, C. & Hope, W. The, EUCAST Afst. Technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 18, E246–E247 (2012).
Arendrup, M.C. et al. EUCAST definitive document EDef 9.3: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents forconidia-forming moulds. EUCAST, Växjö, Sweden. EUCAST Definitive Document EDef 9.3 http://www.aspergillus.org.uk/sites/default/files/pictures/Lab_protocols/EUCAST_E_Def_9_3_Mould_testing_definitive_0.pdf (2015).
Müller, C., Staudacher, V., Krauss, J., Giera, M. & Bracher, F. A convenient cellular assay for the identification of the molecular target of ergosterol biosynthesis inhibitors and quantification of their effects on total ergosterol biosynthesis. Steroids 78, 483–493 (2013).
Müller, C. et al. Fungal sterol C22-desaturase is not an antimycotic target as shown by selective inhibitors and testing on clinical isolates. Steroids 101, 1–6 (2015).
Keller, P. et al. An antifungal benzimidazole derivative inhibits ergosterol biosynthesis and reveals novel sterols. Antimicrob. Agents Chemother. 59, 6296–6307 (2015).
Giera, M., Müller, C. & Bracher, F. Analysis and experimental inhibition of distal cholesterol biosynthesis. Chromatographia 78, 343–358 (2014).
Gerst, N., Ruan, B., Pang, J., Wilson, W.K. & Schroepfer, G.J. An updated look at the analysis of unsaturated C27 sterols by gas chromatography and mass spectrometry. J. Lipid Res. 38, 1685–1701 (1997).
Goad, L.J. & Akihisa, T. Mass spectrometry of sterols in Analysis of Sterols 152–196 (Springer Netherlands, 1997).
Zhao, Y. et al. Expression turnover profiling to monitor the antifungal activities of amphotericin B, voriconazole, and micafungin against Aspergillus fumigatus. Antimicrob. Agents Chemother. 56, 2770–2772 (2012).
Sun, N. et al. Azole susceptibility and transcriptome profiling in Candida albicans mitochondrial electron transport chain complex I mutants. Antimicrob. Agents Chemother. 57, 532–542 (2013).
Burger-Kentischer, A. et al. A screening assay based on host-pathogen interaction models identifies a set of novel antifungal benzimidazole derivatives. Antimicrob. Agents Chemother. 55, 4789–4801 (2011).
Alcazar-Fuoli, L. & Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 3, 439 (2013).
Goad, L.J. & Akihisa, T. Analysis of Sterols 1st edn. (Blackie Academic & Professional, 1997).
Nes, W.D. in Analysis of Sterols and Other Biologically Significant Steroids (ed. Edward J. Parish) iv (Academic Press, 1989).
Renard, D., Perruchon, J., Giera, M., Müller, J. & Bracher, F. Side chain azasteroids and thiasteroids as sterol methyltransferase inhibitors in ergosterol biosynthesis. Bioorg. Med. Chem. 17, 8123–8137 (2009).
Burbiel, J. & Bracher, F. Azasteroids as antifungals. Steroids 68, 587–594 (2003).
Kristan, K. & Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 129, 79–91 (2012).
Matyash, V., Liebisch, G., Kurzchalia, T.V., Shevchenko, A. & Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 (2008).
Giera, M., Plössl, F. & Bracher, F. Fast and easy in vitro screening assay for cholesterol biosynthesis inhibitors in the post-squalene pathway. Steroids 72, 633–642 (2007).
Giera, M. & Bracher, F. First total synthesis of ergosta-5,8-dien-3β-ol. Sci. Pharm. 76, 599–604 (2008).
Kloos, D. et al. Comprehensive gas chromatography–electron ionisation mass spectrometric analysis of fatty acids and sterols using sequential one-pot silylation: quantification and isotopologue analysis. Rapid Commun. Mass Spectrom. 28, 1507–1514 (2014).
Gsaller, F. et al. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAAT binding complex. PLoS Pathog. 12, e1005775 (2016).
Balkis, M.M., Leidich, S.D., Mukherjee, P.K. & Ghannoum, M.A. Mechanisms of fungal resistance. Drugs 62, 1025–1040 (2012).
Wayne, P.A. M38-A2. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd edn. Clinical and Laboratory Standards Institute. http://shop.clsi.org/site/Sample_pdf/M38A2_sample.pdf (2008).
Xu, L. & Porter, N.A. Reactivities and products of free radical oxidation of cholestadienols. J. Am. Chem. Soc. 136, 5443–5450 (2014).
Plössl, F., Giera, M. & Bracher, F. Multiresidue analytical method using dispersive solid-phase extraction and gas chromatography/ion trap mass spectrometry to determine pharmaceuticals in whole blood. J. Chromatogr. A 1135, 19–26 (2006).
Goad, L.J. & Akihisa, T. Gas—liquid chromatography of sterols. in Analysis of Sterols 115–143 (Springer Netherlands, 1997).
Alcazar-Fuoli, L. et al. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 73, 339–347 (2008).
Xu, S., Norton, R.A., Crumley, F.G. & Nes, W.D. Comparison of the chromatographic properties of sterols, select additional steroids and triterpenoids: gravity-flow column liquid chromatography, thin-layer chromatography, gas—liquid chromatography and high-performance liquid chromatography. J. Chromatogr. A 452, 377–398 (1988).
Itoh, T., Sica, D. & Djerassi, C. Minor and trace sterols in marine invertebrates. Part 35. Isolation and structure elucidation of seventy-four sterols from the sponge Axinella cannabina. J Chem. Soc., Perkin Trans. 1, 147–153 (1983).
Lorenz, R.T., Fenner, G., Parks, L.W. & Haeckler, K. 2 - Analysis of steryl esters A2 - Nes, W. David. in Analysis of Sterols and Other Biologically Significant Steroids (ed. Edward J. Parish) 33–47 (Academic Press, 1989).
Giera, M., Renard, D., Plössl, F. & Bracher, F. Lathosterol side chain amides—a new class of human lathosterol oxidase inhibitors. Steroids 73, 299–308 (2008).
Gachotte, D. et al. Characterization of the Saccharomyces cerevisiae ERG27 gene encoding the 3-keto reductase involved in C-4 sterol demethylation. Proc. Natl. Acad. Sci. USA 96, 12655–12660 (1999).
Vanden Bossche, H. et al. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob. Agents Chemother. 37, 2101–2105 (1993).
Ruan, B. et al. Alternative pathways of sterol synthesis in yeast: use of C27 sterol tracers to study aberrant double-bond migrations and evaluate their relative importance. Steroids 67, 1109–1119 (2002).
Georgopapadakou, N.H. Antifungals: mechanism of action and resistance, established and novel drugs. Curr. Opin. Microbiol. 1, 547–557 (1998).
Nes, W.R. A comparison of methods for the identification of sterols. Methods Enzymol. 111, 3–37 1985.
Odds, F.C., Brown, A.J.P. & Gow, N.A.R. Antifungal agents: mechanisms of action. Trends Microbiol. 11, 272–279 (2003).
Goldman, R.C., Zakula, D., Capobianco, J.O., Sharpe, B.A. & Griffin, J.H. Inhibition of 2,3-oxidosqualene-lanosterol cyclase in Candida albicans by pyridinium ion-based inhibitors. Antimicrob. Agents Chemother. 40, 1044–1047 (1996).
Polak, A. Mode of action of morpholine derivatives. Ann. N. Y. Acad. Sci. 544, 221–228 (1988).
Helliwell, S.B. et al. FR171456 is a specific inhibitor of mammalian NSDHL and yeast Erg26p. Nat. Commun. 6, 8613 (2015).
Kuchta, T. et al. Inhibition of sterol 4-demethylation in Candida albicans by 6-amino-2-n-pentylthiobenzothiazole, a novel mechanism of action for an antifungal agent. Antimicrob. Agents Chemother. 39, 1538–1541 (1995).
Goldstein, A.S. Synthesis and bioevaluation of Δ7-5-desaturase inhibitors, an enzyme late in the biosynthesis of the fungal sterol ergosterol. J. Med. Chem. 39, 5092–5099 (1996).
Pierce, H.D., Pierce, A.M., Srinivasan, R., Unrau, A.M. & Oehlschlager, A.C. Azasterol inhibitors in yeast. Biochim. Biophys. Acta 529, 429–437 (1978).
Acknowledgements
We thank J. Müller for his contribution to the early development of this protocol. Further, we thank EMC microcollections for providing the investigational drug EMC120B12.
Author information
Authors and Affiliations
Contributions
C.M., U.B. and M.G. carried out the experiments. C.M., F.B. and M.G. designed the protocol. All authors contributed to writing the protocol.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 (PDF 4485 kb)
Supplementary Data
Supplementary Data. (ZIP 64 kb)
Rights and permissions
About this article
Cite this article
Müller, C., Binder, U., Bracher, F. et al. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography–mass spectrometry. Nat Protoc 12, 947–963 (2017). https://doi.org/10.1038/nprot.2017.005
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.005
This article is cited by
-
Integration of (S)-2,3-oxidosqualene enables E. coli to become Iron Man E. coli with improved overall tolerance
Biotechnology for Biofuels and Bioproducts (2023)
-
Targeting fungal membrane homeostasis with imidazopyrazoindoles impairs azole resistance and biofilm formation
Nature Communications (2022)
-
Transcriptional responses of Candida glabrata biofilm cells to fluconazole are modulated by the carbon source
npj Biofilms and Microbiomes (2020)
-
Analysis of brain metabolites by gas chromatography–mass spectrometry reveals the risk–benefit concerns of prednisone in MRL/lpr lupus mice
Inflammopharmacology (2020)
-
A gas chromatography–mass spectrometry-based whole-cell screening assay for target identification in distal cholesterol biosynthesis
Nature Protocols (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.