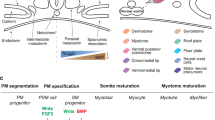
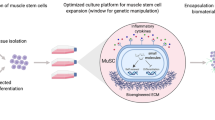
Abstract
Progress toward finding a cure for muscle diseases has been slow because of the absence of relevant cellular models and the lack of a reliable source of muscle progenitors for biomedical investigation. Here we report an optimized serum-free differentiation protocol to efficiently produce striated, millimeter-long muscle fibers together with satellite-like cells from human pluripotent stem cells (hPSCs) in vitro. By mimicking key signaling events leading to muscle formation in the embryo, in particular the dual modulation of Wnt and bone morphogenetic protein (BMP) pathway signaling, this directed differentiation protocol avoids the requirement for genetic modifications or cell sorting. Robust myogenesis can be achieved in vitro within 1 month by personnel experienced in hPSC culture. The differentiating culture can be subcultured to produce large amounts of myogenic progenitors amenable to numerous downstream applications. Beyond the study of myogenesis, this differentiation method offers an attractive platform for the development of relevant in vitro models of muscle dystrophies and drug screening strategies, as well as providing a source of cells for tissue engineering and cell therapy approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chal, J. et al. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 33, 962–969 (2015).
Rohwedel, J. et al. Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: developmentally regulated expression of myogenic determination genes and functional expression of ionic currents. Dev. Biol. 164, 87–101 (1994).
Wobus, A.M. Potential of embryonic stem cells. Mol. Aspects Med. 22, 149–164 (2001).
Greco, T.L. et al. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 10, 313–324 (1996).
Aulehla, A. et al. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat. Cell Biol. 10, 186–193 (2008).
Dunty, W.C. Jr. et al. Wnt3a/beta-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 135, 85–94 (2008).
Yamaguchi, T.P., Takada, S., Yoshikawa, Y., Wu, N. & McMahon, A.P. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 13, 3185–3190 (1999).
Chal, J. & Pourquie, O. Patterning and differentiation of the vertebrate spine. Cold Spring Harb. Monograph 41–116 http://dx.doi.org/10.1101/087969825.53.41 (2009).
Yoshikawa, Y., Fujimori, T., McMahon, A.P. & Takada, S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev. Biol. 183, 234–242 (1997).
Chapman, D.L., Agulnik, I., Hancock, S., Silver, L.M. & Papaioannou, V.E. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev. Biol. 180, 534–542 (1996).
Galceran, J., Farinas, I., Depew, M.J., Clevers, H. & Grosschedl, R. Wnt3a-/--like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev. 13, 709–717 (1999).
Lindsley, R.C., Gill, J.G., Kyba, M., Murphy, T.L. & Murphy, K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133, 3787–3796 (2006).
Gadue, P., Huber, T.L., Paddison, P.J. & Keller, G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 16806–16811 (2006).
Sumi, T., Tsuneyoshi, N., Nakatsuji, N. & Suemori, H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 (2008).
Nakanishi, M. et al. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 23, 114–122 (2009).
Xu, C. et al. A zebrafish embryo culture system defines factors that promote vertebrate myogenesis across species. Cell 155, 909–921 (2013).
Borchin, B., Chen, J. & Barberi, T. Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Rep. 1, 620–631 (2013).
Shelton, M. et al. Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Rep. 3, 516–529 (2014).
Hwang, Y. et al. WNT3A promotes myogenesis of human embryonic stem cells and enhances in vivo engraftment. Sci. Rep. 4, 5916 (2014).
Aulehla, A. et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 4, 395–406 (2003).
Geetha-Loganathan, P., Nimmagadda, S., Scaal, M., Huang, R. & Christ, B. Wnt signaling in somite development. Ann. Anat. 190, 208–222 (2008).
Tonegawa, A., Funayama, N., Ueno, N. & Takahashi, Y. Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development 124, 1975–1984 (1997).
Hirsinger, E. et al. Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development 124, 4605–4614 (1997).
McMahon, J.A. et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12, 1438–1452 (1998).
Reshef, R., Maroto, M. & Lassar, A.B. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 12, 290–303 (1998).
Streit, A. & Stern, C.D. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech. Dev. 85, 85–96 (1999).
Miura, S., Davis, S., Klingensmith, J. & Mishina, Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development 133, 3767–3775 (2006).
Sela-Donenfeld, D. & Kalcheim, C. Localized BMP4-noggin interactions generate the dynamic patterning of noggin expression in somites. Dev. Biol. 246, 311–328 (2002).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Umeda, K. et al. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci. Rep. 2, 455 (2012).
Adelman, C.A., Chattopadhyay, S. & Bieker, J.J. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development 129, 539–549 (2002).
Nostro, M.C., Cheng, X., Keller, G.M. & Gadue, P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell 2, 60–71 (2008).
Lengerke, C. et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell 2, 72–82 (2008).
Orlova, V.V., Chuva de Sousa Lopes, S. & Valdimarsdottir, G. BMP-SMAD signaling: from pluripotent stem cells to cardiovascular commitment. Cytokine Growth Factor Rev. 27, 55–63 (2016).
Vivarelli, E. & Cossu, G. Neural control of early myogenic differentiation in cultures of mouse somites. Dev. Biol. 117, 319–325 (1986).
Cossu, G., Kelly, R., Di Donna, S., Vivarelli, E. & Buckingham, M. Myoblast differentiation during mammalian somitogenesis is dependent upon a community effect. Proc. Natl. Acad. Sci. USA 92, 2254–2258 (1995).
Buffinger, N. & Stockdale, F.E. Myogenic specification of somites is mediated by diffusible factors. Dev. Biol. 169, 96–108 (1995).
Rong, P.M., Teillet, M.A., Ziller, C. & Le Douarin, N.M. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development 115, 657–672 (1992).
Miller, J.B., Everitt, E.A., Smith, T.H., Block, N.E. & Dominov, J.A. Cellular and molecular diversity in skeletal muscle development: news from in vitro and. Bioessays 15, 191–196 (1993).
Neville, C., Rosenthal, N., McGrew, M., Bogdanova, N. & Hauschka, S. Skeletal muscle cultures. Methods Cell Biol. 52, 85–116 (1997).
Danoviz, M.E. & Yablonka-Reuveni, Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol. Biol. 798, 21–52 (2012).
Miller, J.B. & Stockdale, F.E. Developmental origins of skeletal muscle fibers: clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proc. Natl. Acad. Sci. USA 83, 3860–3864 (1986).
Rutz, R. & Hauschka, S. Clonal analysis of vertebrate myogenesis. VII. Heritability of muscle colony type through sequential subclonal passages in vitro. Dev. Biol. 91, 103–110 (1982).
Lian, X. et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 3, 804–816 (2014).
Tesar, P.J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007).
Shelton, M., Kocharyan, A., Liu, J., Skerjanc, I.S. & Stanford, W.L. Robust generation and expansion of skeletal muscle progenitors and myocytes from human pluripotent stem cells. Methods 101, 73–84 (2015).
Mahmood, A., Harkness, L., Schroder, H.D., Abdallah, B.M. & Kassem, M. Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. J. Bone Miner. Res. 25, 1216–1233 (2010).
Hosoyama, T., McGivern, J.V., Van Dyke, J.M., Ebert, A.D. & Suzuki, M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl. Med. 3, 564–574 (2014).
Maffioletti, S.M. et al. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat. Protoc. 10, 941–958 (2015).
Shoji, E., Woltjen, K. & Sakurai, H. Directed myogenic differentiation of human induced pluripotent stem cells. Methods Mol. Biol. 1353, 89–99 (2016).
Albini, S. & Puri, P.L. Generation of myospheres from hESCs by epigenetic reprogramming. J. Vis. Exp. 88, e51243 (2014).
Gerli, M.F., Maffioletti, S.M., Millet, Q. & Tedesco, F.S. Transplantation of induced pluripotent stem cell-derived mesoangioblast-like myogenic progenitors in mouse models of muscle regeneration. J. Vis. Exp. 83, e50532 (2014).
Darabi, R. & Perlingeiro, R.C. Derivation of skeletal myogenic precursors from human pluripotent stem cells using conditional expression of PAX7. Methods Mol. Biol. 1357, 423–439 (2016).
Stavropoulos, M.E., Mengarelli, I. & Barberi, T. Differentiation of multipotent mesenchymal precursors and skeletal myoblasts from human embryonic stem cells. Curr. Protoc. Stem Cell Biol. Chapter 1, Unit 1F 8 (2009).
Filareto, A. et al. An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells. Nat. Commun. 4, 1549 (2013).
Quattrocelli, M. et al. Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs. J. Pathol. 223, 593–603 (2011).
Barberi, T. et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 13, 642–648 (2007).
Beers, J. et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 7, 2029–2040 (2012).
Baharvand, H., Salekdeh, G.H., Taei, A. & Mollamohammadi, S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protoc. 5, 588–594 (2010).
Marti, M. et al. Characterization of pluripotent stem cells. Nat. Protoc. 8, 223–253 (2013).
Schwartz, P.H., Brick, D.J., Nethercott, H.E. & Stover, A.E. Traditional human embryonic stem cell culture. Methods Mol. Biol. 767, 107–123 (2011).
Lin, S. & Talbot, P. Methods for culturing mouse and human embryonic stem cells. Methods Mol. Biol. 690, 31–56 (2011).
van den Brink, S.C. et al. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231–4242 (2014).
Gouti, M. et al. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12, e1001937 (2014).
Rohwedel, J., Guan, K., Hegert, C. & Wobus, A.M. Embryonic stem cells as an in vitro model for mutagenicity, cytotoxicity and embryotoxicity studies: present state and future prospects. Toxicology In Vitro 15, 741–753 (2001).
Giobbe, G.G. et al. Functional differentiation of human pluripotent stem cells on a chip. Nat. Methods 12, 637–640 (2015).
Park, D., Lim, J., Park, J.Y. & Lee, S.H. Concise review: stem cell microenvironment on a chip: current technologies for tissue engineering and stem cell biology. Stem Cells Transl. Med. 4, 1352–1368 (2015).
Ostrovidov, S. et al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 20, 403–436 (2014).
Cheng, C.W., Solorio, L.D. & Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 32, 462–484 (2014).
Dennis, R.G. & Kosnik, P.E. II. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell. Dev. Biol. Anim. 36, 327–335 (2000).
Bian, W. & Bursac, N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials 30, 1401–1412 (2009).
Juhas, M. & Bursac, N. Engineering skeletal muscle repair. Curr. Opin. Biotechnol. 24, 880–886 (2013).
Sakar, M.S. et al. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12, 4976–4985 (2012).
Neal, D., Sakar, M.S., Ong, L.L. & Harry Asada, H. Formation of elongated fascicle-inspired 3D tissues consisting of high-density, aligned cells using sacrificial outer molding. Lab Chip 14, 1907–1916 (2014).
Grosberg, A. et al. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J. Pharmacol. Toxicol. Methods 65, 126–135 (2012).
Benam, K.H. et al. Engineered in vitro disease models. Ann. Rev. Pathol. 10, 195–262 (2015).
Vandenburgh, H. Functional assessment and tissue design of skeletal muscle. Ann. NY Acad. Sci. 961, 201–202 (2002).
Vandenburgh, H., Shansky, J., Del Tatto, M. & Chromiak, J. Organogenesis of skeletal muscle in tissue culture. Methods Mol. Med. 18, 217–225 (1999).
Vandenburgh, H. et al. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J. 23, 3325–3334 (2009).
Vandenburgh, H. et al. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37, 438–447 (2008).
Demestre, M. et al. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 15, 328–336 (2015).
Das, M., Rumsey, J.W., Bhargava, N., Stancescu, M. & Hickman, J.J. Skeletal muscle tissue engineering: a maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression. Biomaterials 30, 5392–5402 (2009).
Das, M., Rumsey, J.W., Bhargava, N., Stancescu, M. & Hickman, J.J. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials 31, 4880–4888 (2010).
Guo, X. et al. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng. Part C Methods 16, 1347–1355 (2010).
Askanas, V. et al. Accumulation of CK-MM is impaired in innervated and contracting cultured muscle fibers of Duchenne muscular dystrophy patients. Life Sci. 41, 927–933 (1987).
Smith, A.S., Long, C.J., Pirozzi, K. & Hickman, J.J. A functional system for high-content screening of neuromuscular junctions. Technology 1, 37–48 (2013).
Thomson, S.R. et al. Morphological characteristics of motor neurons do not determine their relative susceptibility to degeneration in a mouse model of severe spinal muscular atrophy. PloS One 7, e52605 (2012).
Puttonen, K.A. et al. Generation of functional neuromuscular junctions from human pluripotent stem cell lines. Front. Cell. Neurosci. 9, 473 (2015).
Rohwedel, J. et al. Formation of postsynaptic-like membranes during differentiation of embryonic stem cells in vitro. Exp. Cell Res. 239, 214–225 (1998).
Kostrominova, T.Y., Calve, S., Arruda, E.M. & Larkin, L.M. Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro. Histol. Histopathol. 24, 541–550 (2009).
Lui, P.P. Stem cell technology for tendon regeneration: current status, challenges, and future research directions. Stem Cells Cloning 7, 163–174 (2015).
Juhas, M., Engelmayr, G.C. Jr., Fontanella, A.N., Palmer, G.M. & Bursac, N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation. Proc. Natl. Acad. Sci. USA 111, 5508–5513 (2014).
Gholobova, D. et al. Endothelial network formation within human tissue-engineered skeletal muscle. Tissue Eng. Part A 21, 2548–2558 (2015).
Carosio, S. et al. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET). Sci. Rep. 3, 1420 (2013).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Dodson, M.V., Vierck, J.L., Hossner, K.L., Byrne, K. & McNamara, J.P. The development and utility of a defined muscle and fat co-culture system. Tissue Cell 29, 517–524 (1997).
Shoji, E. et al. Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci. Rep. 5, 12831 (2015).
Abujarour, R. et al. Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl. Med. 3, 149–160 (2014).
Yasuno, T. et al. Functional analysis of iPSC-derived myocytes from a patient with carnitine palmitoyltransferase II deficiency. Biochem. Biophys. Res. Commun. 448, 175–181 (2014).
Tanaka, A. et al. Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PloS One 8, e61540 (2013).
Kawagoe, S. et al. Generation of induced pluripotent stem (iPS) cells derived from a murine model of Pompe disease and differentiation of Pompe-iPS cells into skeletal muscle cells. Mol. Genet. Metab. 104, 123–128 (2011).
Young, C.S. et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18, 533–540 (2016).
Tran, T., Andersen, R., Sherman, S.P. & Pyle, A.D. Insights into skeletal muscle development and applications in regenerative medicine. Int. Rev. Cell Mol. Biol. 300, 51–83 (2013).
Li, H.L. et al. Precise correction of the dystrophin gene in Duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep. 4, 143–154 (2015).
Yokota, T., Pistilli, E., Duddy, W. & Nagaraju, K. Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin. Biol. Ther. 7, 831–842 (2007).
Kazuki, Y. et al. Complete genetic correction of iPS cells from Duchenne muscular dystrophy. Mol. Ther. 18, 386–393 (2010).
van Deutekom, J.C. et al. Local dystrophin restoration with antisense oligonucleotide PRO051. New Engl. J. Med. 357, 2677–2686 (2007).
Skuk, D. & Tremblay, J.P. Intramuscular cell transplantation as a potential treatment of myopathies: clinical and preclinical relevant data. Expert Opin. Biol. Ther. 11, 359–374 (2011).
Ostrovidov, S. et al. Stem cell differentiation toward the myogenic lineage for muscle tissue regeneration: a focus on muscular dystrophy. Stem Cell Rev. 11, 866–884 (2015).
Bursac, N., Juhas, M. & Rando, T.A. Synergizing engineering and biology to treat and model skeletal muscle injury and disease. Ann. Rev. Biomed. Eng. 17, 217–242 (2015).
Liao, H. & Zhou, G.Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B Rev. 15, 319–331 (2009).
Koning, M., Harmsen, M.C., van Luyn, M.J. & Werker, P.M. Current opportunities and challenges in skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 3, 407–415 (2009).
Tourovskaia, A., Figueroa-Masot, X. & Folch, A. Long-term microfluidic cultures of myotube microarrays for high-throughput focal stimulation. Nat. Protoc. 1, 1092–1104 (2006).
Reimann, J. et al. Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res. 315, 233–242 (2004).
Kottlors, M. & Kirschner, J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res. 340, 541–548 (2010).
Lepper, C., Partridge, T.A. & Fan, C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 (2011).
Sambasivan, R. et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 (2011).
Murphy, M.M., Lawson, J.A., Mathew, S.J., Hutcheson, D.A. & Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637 (2011).
Roca, I., Requena, J., Edel, M.J. & Alvarez-Palomo, A.B. Myogenic precursors from iPS cells for skeletal muscle cell replacement therapy. J. Clin. Med. 4, 243–259 (2015).
Mendell, J.R. et al. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. New Engl. J. Med. 333, 832–838 (1995).
Rando, T.A., Pavlath, G.K. & Blau, H.M. The fate of myoblasts following transplantation into mature muscle. Exp. Cell Res. 220, 383–389 (1995).
Gussoni, E., Blau, H.M. & Kunkel, L.M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 3, 970–977 (1997).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Collins, C.A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005).
Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. & Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008).
Boldrin, L. & Morgan, J.E. Human satellite cells: identification on human muscle fibres. PLoS Curr. 3, RRN1294 (2011).
Marg, A. et al. Human satellite cells have regenerative capacity and are genetically manipulable. J. Clin. Invest. 124, 4257–4265 (2014).
Xu, X. et al. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep. 5, 419–434 (2015).
Gilbert, P.M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Tierney, M.T. et al. Autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Rep. 1–13 (2016).
Charville, G.W. et al. Ex vivo expansion and in vivoself-renewal of human muscle stem cells. Stem Cell Rep. 5, 621–632 (2015).
Meng, J., Adkin, C.F., Xu, S.W., Muntoni, F. & Morgan, J.E. Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PloS One 6, e17454 (2011).
Tajbakhsh, S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J. Intern. Med. 266, 372–389 (2009).
Baker, R.K. & Lyons, G.E. Embryonic stem cells and in vitro muscle development. Curr. Top. Dev. Biol. 33, 263–279 (1996).
Darabi, R., Santos, F.N. & Perlingeiro, R.C. The therapeutic potential of embryonic and adult stem cells for skeletal muscle regeneration. Stem Cell Rev. 4, 217–225 (2008).
Salani, S. et al. Generation of skeletal muscle cells from embryonic and induced pluripotent stem cells as an in vitro model and for therapy of muscular dystrophies. J. Cell. Mol. Med. 16, 1353–1364 (2012).
Vilquin, J.T. Converting pathological cells to therapeutic ones: an odyssey through pluripotency. Mol. Ther. 20, 2012–2014 (2012).
Swierczek, B., Ciemerych, M.A. & Archacka, K. From pluripotency to myogenesis: a multistep process in the dish. J. Muscle Res. Cell Motil. 36, 363–375 (2015).
Grabowska, I., Archacka, K., Czerwinska, A.M., Krupa, M. & Ciemerych, M.A. Mouse and human pluripotent stem cells and the means of their myogenic differentiation. Results Probl. Cell Differ. 55, 321–356 (2012).
Wilschut, K.J., Ling, V.B. & Bernstein, H.S. Concise review: stem cell therapy for muscular dystrophies. Stem Cells Transl. Med. 1, 833–842 (2012).
Abujarour, R. & Valamehr, B. Generation of skeletal muscle cells from pluripotent stem cells: advances and challenges. Front. Cell Dev. Biol. 3, 29 (2015).
Dekel, I., Magal, Y., Pearson-White, S., Emerson, C.P. & Shani, M. Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol. 4, 217–224 (1992).
Shani, M. et al. The consequences of a constitutive expression of MyoD1 in ES cells and mouse embryos. Symp. Soc. Exp. Biol. 46, 19–36 (1992).
Davis, R.L., Weintraub, H. & Lassar, A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Comai, G. & Tajbakhsh, S. Molecular and cellular regulation of skeletal myogenesis. Curr. Top. Dev. Biol. 110, 1–73 (2014).
Darabi, R. et al. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat. Med. 14, 134–143 (2008).
Rao, L. et al. Highly efficient derivation of skeletal myotubes from human embryonic stem cells. Stem Cell Rev. 8, 1109–1119 (2012).
Goudenege, S. et al. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 20, 2153–2167 (2012).
Albini, S. et al. Epigenetic reprogramming of human embryonic stem cells into skeletal muscle cells and generation of contractile myospheres. Cell Rep. 3, 661–670 (2013).
Darabi, R. et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619 (2012).
Fougerousse, F. et al. Six and Eya expression during human somitogenesis and MyoD gene family activation. J. Muscle Res. Cell Motil. 23, 255–264 (2002).
Goulding, M.D., Chalepakis, G., Deutsch, U., Erselius, J.R. & Gruss, P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 10, 1135–1147 (1991).
Jostes, B., Walther, C. & Gruss, P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 33, 27–37 (1990).
Gerard, M. et al. PAX-genes expression during human embryonic development, a preliminary report. C R Acad Sci III 318, 57–66 (1995).
Terzic, J. & Saraga-Babic, M. Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Int. J. Dev. Biol. 43, 501–508 (1999).
Skoglund, G. et al. Physiological and ultrastructural features of human induced pluripotent and embryonic stem cell-derived skeletal myocytes in vitro. Proc. Natl. Acad. Sci. USA 111, 8275–8280 (2014).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Peault, B. et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 15, 867–877 (2007).
Dellavalle, A. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267 (2007).
Tedesco, F.S. et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Science Transl. Med. 4, 140ra189 (2012).
Moretti, A., Laugwitz, K.L., Dorn, T., Sinnecker, D. & Mummery, C. Pluripotent stem cell models of human heart disease. Cold Spring Harbor Perspectives in Medicine 3, a014027 (2013).
Robertson, C., Tran, D.D. & George, S.C. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31, 829–837 (2013).
Bock, C. et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Boulting, G.L. et al. A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 29, 279–286 (2011).
Halme, D.G. & Kessler, D.A. FDA regulation of stem-cell-based therapies. New Engl. J. Med. 355, 1730–1735 (2006).
Vandenburgh, H. High-content drug screening with engineered musculoskeletal tissues. Tissue Eng. Part B Rev. 16, 55–64 (2010).
Desbordes, S.C. & Studer, L. Adapting human pluripotent stem cells to high-throughput and high-content screening. Nat. Protoc. 8, 111–130 (2013).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Kleinman, H.K. et al. Basement membrane complexes with biological activity. Biochemistry 25, 312–318 (1986).
Hauschka, S.D. & Konigsberg, I.R. The influence of collagen on the development of muscle clones. Proc. Natl. Acad. Sci. USA 55, 119–126 (1966).
Kuhl, U., Ocalan, M., Timpl, R. & von der Mark, K. Role of laminin and fibronectin in selecting myogenic versus fibrogenic cells from skeletal muscle cells in vitro. Dev. Biol. 117, 628–635 (1986).
von der Mark, K. & Ocalan, M. Antagonistic effects of laminin and fibronectin on the expression of the myogenic phenotype. Differentiation 40, 150–157 (1989).
Maley, M.A., Davies, M.J. & Grounds, M.D. Extracellular matrix, growth factors, genetics: their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Exp. Cell Res. 219, 169–179 (1995).
Pinset, C. & Whalen, R.G. Induction of myogenic differentiation in serum-free medium does not require DNA synthesis. Dev. Biol. 108, 284–289 (1985).
Goto, S., Miyazaki, K., Funabiki, T. & Yasumitsu, H. Serum-free culture conditions for analysis of secretory proteinases during myogenic differentiation of mouse C2C12 myoblasts. Anal. Biochem. 272, 135–142 (1999).
Reiss, K. & Korohoda, W. The formation of myotubes in cultures of chick embryo myogenic cells in serum-free medium is induced by the insulin pulse treatment. Folia Histochem. Cytobiol. 26, 133–141 (1988).
Herrmann, B.G., Labeit, S., Poustka, A., King, T.R. & Lehrach, H. Cloning of the T gene required in mesoderm formation in the mouse. Nature 343, 617–622 (1990).
Kispert, A. & Herrmann, B.G. Immunohistochemical analysis of the Brachyury protein in wild-type and mutant mouse embryos. Dev. Biol. 161, 179–193 (1994).
Yoon, J.K. & Wold, B. The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev. 14, 3204–3214 (2000).
Yoon, J.K., Moon, R.T. & Wold, B. The bHLH class protein pMesogenin1 can specify paraxial mesoderm phenotypes. Dev. Biol. 222, 376–391 (2000).
Sassoon, D. et al. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature 341, 303–307 (1989).
Braun, T. & Arnold, H.H. ES-cells carrying two inactivated myf-5 alleles form skeletal muscle cells: activation of an alternative myf-5-independent differentiation pathway. Dev. Biol. 164, 24–36 (1994).
Yablonka-Reuveni, Z. & Paterson, B.M. MyoD and myogenin expression patterns in cultures of fetal and adult chicken myoblasts. J. Histochem. Cytochem. 49, 455–462 (2001).
Lyons, G.E., Ontell, M., Cox, R., Sassoon, D. & Buckingham, M. The expression of myosin genes in developing skeletal muscle in the mouse embryo. J. Cell Biol. 111, 1465–1476 (1990).
Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786 (2000).
Zammit, P.S. et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119, 1824–1832 (2006).
McCain, M.L., Agarwal, A., Nesmith, H.W., Nesmith, A.P. & Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 35, 5462–5471 (2014).
Pasqualini, F.S., Sheehy, S.P., Agarwal, A., Aratyn-Schaus, Y. & Parker, K.K. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Rep. 4, 340–347 (2015).
Acknowledgements
We thank all coauthors of the original article describing this technology for their initial support and contribution. We thank G. Guevara for lab assistance. We thank C. Fugier and F. Bousson for comments and feedback. We are grateful to L. Daheron and members of the Pourquié laboratory for comments. We thank A. Freismuth and M. Humbert from the IGBMC cell culture service for hiPSC culture assistance. This work was supported by an advanced grant from the European Research Council to O.P., by the FP7 EU grant Plurimes (agreement no. 602423 to O.P.), by a strategic grant from the French Muscular Dystrophy Association (AFM) to O.P. and by the INGESTEM project (ANR).
Author information
Authors and Affiliations
Contributions
J.C. and Z.A.T. designed and performed the experiments and protocol optimizations, analyzed data and coordinated the project. M.H. and S.A. carried out hPSC differentiation and protocol optimization under the supervision of J.C. B.G. carried out some hPSC optimization experiments under the supervision of Z.A.T. A.H. contributed to hPSC cell culture analysis and data interpretation. T.C. contributed to hPSC culture differentiation and analysis. A.P.N. manufactured micropatterned substrates and performed the fiber structural analysis. K.K.P. provided expertise. O.P. supervised the overall project. J.C., Z.A.T. and O.P. performed the final data analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The work described in this article is partially covered by patent application no. PCT/EP2012/066793 (publication no. WO2013030243 A1). O.P. and J.C. are cofounders and shareholders of Anagenesis Biotechnologies, a startup company specializing in the production of muscle cells in vitro for cell therapy and drug screening.
Rights and permissions
About this article
Cite this article
Chal, J., Al Tanoury, Z., Hestin, M. et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc 11, 1833–1850 (2016). https://doi.org/10.1038/nprot.2016.110
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.110
This article is cited by
-
Motor neurons and endothelial cells additively promote development and fusion of human iPSC-derived skeletal myocytes
Skeletal Muscle (2024)
-
Extracellular matrix: the critical contributor to skeletal muscle regeneration—a comprehensive review
Inflammation and Regeneration (2023)
-
Simple and efficient differentiation of human iPSCs into contractible skeletal muscles for muscular disease modeling
Scientific Reports (2023)
-
Metabolic regulation of species-specific developmental rates
Nature (2023)
-
Stem cell-based strategies and challenges for production of cultivated meat
Nature Food (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.