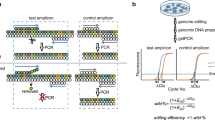
Abstract
Genome editing using designer nucleases such as transcription activator-like effector nucleases (TALENs) or clustered regularly interspersed short palindromic repeats (CRISPR)-Cas9 nucleases is an emerging technology in basic and applied research. Whereas the application of editing tools, namely CRISPR-Cas9, has recently become very straightforward, quantification of resulting gene knockout rates still remains a bottleneck. This is particularly true if the product of a targeted gene is not easily detectable. To address this problem, we devised a novel gene-editing frequency digital PCR (GEF-dPCR) technique. GEF-dPCR exploits two differently labeled probes that are placed within one amplicon at the gene-editing target site to simultaneously detect wild-type and nonhomologous end-joining (NHEJ)-affected alleles. Taking advantage of the principle of dPCR, this enables concurrent quantification of edited and wild-type alleles in a given sample. We propose that our method is optimal for the monitoring of gene-edited cells in vivo, e.g., in clinical settings. Here we describe preparation, design of primers and probes, and setup and analysis of GEF-dPCR. The setup of GEF-dPCR requires up to 2 weeks (depending on the starting point); once the dPCR has been established, the protocol for sample analysis takes <1 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Ding, Q. et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12, 238–251 (2013).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR-Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Yoshimi, K., Kaneko, T., Voigt, B. & Mashimo, T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR-Cas platform. Nat. Commun. 5, 4240 (2014).
Menon, T. et al. Lymphoid regeneration from gene-corrected SCID-X1 subject-derived iPSCs. Cell Stem Cell 16, 367–372 (2015).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240 (2014).
Lombardo, A. et al. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat. Methods 8, 861–869 (2011).
Bloom, K., Ely, A., Mussolino, C., Cathomen, T. & Arbuthnot, P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol. Ther. 21, 1889–1897 (2013).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815 (2012).
Berdien, B., Mock, U., Atanackovic, D. & Fehse, B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 21, 539–548 (2014).
Mock, U. et al. mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res. 43, 5560–5571 (2015).
Sanjana, N.E. et al. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192 (2012).
Schmid-Burgk, J.L., Schmidt, T., Kaiser, V., Höning, K. & Hornung, V. A ligation-independent cloning technique for high-throughput assembly of transcription activator–like effector genes. Nat. Biotechnol. 31, 76–81 (2012).
Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Miyaoka, Y. et al. Isolation of single-base genome-edited human iPS cells without antibiotic selection. Nat. Methods 11, 291–293 (2014).
Thomas, H.R., Percival, S.M., Yoder, B.K. & Parant, J.M. High-throughput genome editing and phenotyping facilitated by high resolution melting curve analysis. PLoS ONE 9, e114632 (2014).
Baker, M. Digital PCR hits its stride. Nat. Methods 9, 541–544 (2012).
Huggett, J.F., Cowen, S. & Foy, C.A. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin. Chem. 61, 79–88 (2015).
Guschin, D.Y. et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol. Biol. 649, 247–256 (2010).
Perez, E.E. et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26, 808–816 (2008).
Güell, M., Yang, L. & Church, G.M. Genome editing assessment using CRISPR Genome Analyzer (CRISPR-GA). Bioinformatics 30, 2968–2970 (2014).
Schmid-Burgk, J.L. et al. OutKnocker: a web tool for rapid and simple genotyping of DN edited cell lines. Genome Res. 24, 1719–1723 (2014).
Mardis, E.R. Next-generation sequencing platforms. Annu. Rev. Anal. Chem. 6, 287–303 (2013).
Vogelstein, B. & Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 96, 9236–9241 (1999).
Huggett, J.F. et al. The digital MIQE guidelines: minimum information for publication of quantitative digital PCR experiments. Clin. Chem. 59, 892–902 (2013).
Stahl, T., Böhme, M.U., Kröger, N. & Fehse, B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp. Hematol. 43, 462–468 e1 (2015).
Kim, Y., Kweon, J. & Kim, J.-S. TALENs and ZFNs are associated with different mutation signatures. Nat. Methods 10, 185 (2013).
Bae, S., Kweon, J., Kim, H.S. & Kim, J.-S. Microhomology-based choice of Cas9 nuclease target sites. Nat. Methods 11, 705–706 (2014).
Kutyavin, I.V et al. 3′-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28, 655–661 (2000).
Kutner, R.H., Zhang, X.-Y. & Reiser, J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 4, 495–505 (2009).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Stacey, G.N. & Masters, J.R. Cryopreservation and banking of mammalian cell lines. Nat. Protoc. 3, 1981–1989 (2008).
Hall, T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98 (1999).
Nolan, T., Hands, R.E. & Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1, 1559–1582 (2006).
Weissenborn, S.J., Wieland, U., Junk, M. & Pfister, H. Quantification of β-human papillomavirus DNA by real-time PCR. Nat. Protoc. 5, 1–13 (2010).
Acknowledgements
The authors are indebted to T. Sonntag for excellent technical support. In addition, we thank T. Stahl and A. Badbaran for the very valuable discussion. We are very grateful to K. Riecken for critically reading the manuscript and W. Qasim for supporting the generation of the manuscript. This work has been supported by the Deutsche Forschungsgemeinschaft (DFG; SFB841/SP2 to B.F.), the National Institute of Health Research via the Great Ormond Street/Institute of Child Health Biomedical Research Centre (to U.M.) and the German Centre for Infection Research (DZIF).
Author information
Authors and Affiliations
Contributions
U.M. and B.F. developed the technique, performed most experiments, analyzed and interpreted data and wrote the manuscript. I.H. performed the in vivo experiment. All authors have read the manuscript and confirmed their authorship.
Corresponding authors
Ethics declarations
Competing interests
U.M. and B.F. have submitted a patent application (decision pending) for the exemplarily used designer nuclease (CCR5-Uco-TALEN). They have no competing interests regarding the described PCR method. I.H. has no competing financial interests.
Rights and permissions
About this article
Cite this article
Mock, U., Hauber, I. & Fehse, B. Digital PCR to assess gene-editing frequencies (GEF-dPCR) mediated by designer nucleases. Nat Protoc 11, 598–615 (2016). https://doi.org/10.1038/nprot.2016.027
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.027
This article is cited by
-
Quantitative analysis of CRISPR/Cas9-mediated provirus deletion in blue egg layer chicken PGCs by digital PCR
Scientific Reports (2022)
-
Optimisation of a TALE nuclease targeting the HIV co-receptor CCR5 for clinical application
Gene Therapy (2021)
-
Automated production of CCR5-negative CD4+-T cells in a GMP-compatible, clinical scale for treatment of HIV-positive patients
Gene Therapy (2021)
-
CRISPR-based genome editing in primary human pancreatic islet cells
Nature Communications (2021)
-
Detection of genome edits in plants—from editing to seed
In Vitro Cellular & Developmental Biology - Plant (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.