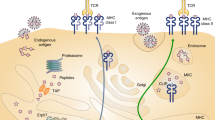
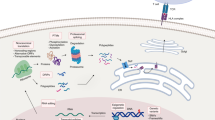
Abstract
Phosphorylation events within cancer cells often become dysregulated, leading to oncogenic signaling and abnormal cell growth. Phosphopeptides derived from aberrantly phosphorylated proteins that are presented on tumors and not on normal tissues by human leukocyte antigen (HLA) class I molecules are promising candidates for future cancer immunotherapies, because they are tumor specific and have been shown to elicit cytotoxic T cell responses. Robust phosphopeptide enrichments that are suitable for low input amounts must be developed to characterize HLA-associated phosphopeptides from clinical samples that are limited by material availability. We present two complementary mass spectrometry–compatible, iron(III)-immobilized metal affinity chromatography (IMAC) methods that use either nitrilotriacetic acid (NTA) or iminodiacetic acid (IDA) in-house-fabricated columns. We developed these protocols to enrich for subfemtomole-level phosphopeptides from cell line and human tissue samples containing picograms of starting material, which is an order of magnitude less material than what is commonly used. In addition, we added a peptide esterification step to increase phosphopeptide specificity from these low-input samples. To date, hundreds of phosphopeptides displayed on melanoma, ovarian cancer, leukemia and colorectal cancer have been identified using these highly selective phosphopeptide enrichment protocols in combination with a program called 'CAD Neutral Loss Finder' that identifies all spectra containing the characteristic neutral loss of phosphoric acid from phosphorylated serine and threonine residues. This methodology enables the identification of HLA-associated phosphopeptides presented by human tissue samples containing as little as nanograms of peptide material in 2 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mohammed, F. et al. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: a molecular basis for the presentation of transformed self. Nat. Immunol. 9, 1236–1243 (2008).
Zarling, A.L. et al. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 103, 14889–14894 (2006).
Engelhard, V.H., Brickner, A.G. & Zarling, A.L. Insights into antigen processing gained by direct analysis of the naturally processed class I MHC associated peptide repertoire. Mol. Immunol. 39, 127–137 (2002).
Zarling, A.L. et al. Phosphorylated peptides are naturally processed and presented by major Histocompatibility complex class I molecules in vivo. J. Exp. Med. 192, 1755–1762 (2000).
Cobbold, M. et al. MHC class I–associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci. Transl. Med. 5, 203ra125 (2013).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Vivier, E., Ugolini, S., Blaise, D., Chabannon, C. & Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 12, 239–252 (2012).
Neefjes, J., Jongsma, M.L.M., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
Hansen, T.H. & Bouvier, M. MHC class I antigen presentation: learning from viral evasion strategies. Nat. Rev. Immunol. 9, 503–513 (2009).
Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ficarro, S.B. et al. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20, 301–305 (2002).
Ficarro, S. et al. Phosphoproteome analysis of capacitated human sperm: evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 278, 11579–11589 (2003).
Olsen, J.V. et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 (2006).
Villen, J. & Gygi, S.P. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat. Protoc. 3, 1630–1638 (2008).
Steen, H., Stensballe, A. & Jensen, O.N. Phosphopeptide purification by IMAC with Fe(III) and Ga(III). Cold Spring Harb. Protoc. 2007, pdb.prot4607 (2007).
Thingholm, T. & Jensen, O. Enrichment and characterization of phosphopeptides by immobilized metal affinity chromatography (IMAC) and mass spectrometry in Phospho-Proteomics: Methods and protocols (ed. Graauw, M.) 527, 47–56 (Humana Press, 2009).
Ye, J. et al. Optimized IMACIMAC protocol for phosphopeptide recovery from complex biological samples. J. Proteome Res. 9, 3561–3573 (2010).
Dunn, J.D., Reid, G.E. & Bruening, M.L. Techniques for phosphopeptide enrichment prior to analysis by mass spectrometry. Mass Spectrom. Rev. 29, 29–54 (2010).
Zhou, H. et al. Robust phosphoproteome enrichment using monodisperse microsphere–based immobilized titanium (IV) ion affinity chromatography. Nat. Protoc. 8, 461–480 (2013).
Thingholm, T.E., Jorgensen, T.J.D., Jensen, O.N. & Larsen, M.R. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 1, 1929–1935 (2006).
Ruprecht, B. et al. Comprehensive and reproducible phosphopeptide enrichment using Fe-IMAC columns. Mol. Cell. Proteomics 14, 205–215 (2015).
Ficarro, S.B. et al. Online nanoflow multidimensional fractionation for high efficiency phosphopeptide analysis. Mol. Cell. Proteomics 10, O111.011064 (2011).
Mertins, P. et al. Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat. Methods 10, 634–637 (2013).
Mertins, P. et al. Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol. Cell. Proteomics 13, 1690–1704 (2014).
Mazanek, M. et al. Titanium dioxide as a chemo-affinity solid phase in offline phosphopeptide chromatography prior to HPLC-MS/MS analysis. Nat. Protoc. 2, 1059–1069 (2007).
Matheron, L., van den Toorn, H., Heck, A.J.R. & Mohammed, S. Characterization of biases in phosphopeptide enrichment by Ti4+-immobilized metal affinity chromatography and TiO2 using a massive synthetic library and human cell digests. Anal. Chem. 86, 8312–8320 (2014).
Zhou, H. et al. Specific phosphopeptide enrichment with immobilized titanium ion affinity chromatography adsorbent for phosphoproteome analysis. J. Proteome Res. 7, 3957–3967 (2008).
Jedrychowski, M.P. et al. Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol. Cell. Proteomics 10, M111.009910 (2011).
Zhang, Y., Ficarro, S., Li, S. & Marto, J. Optimized orbitrap HCD for quantitative analysis of phosphopeptides. J. Am. Soc. Mass Spectrom. 20, 1425–1434 (2009).
Syka, J.E.P., Coon, J.J., Schroeder, M.J., Shabanowitz, J. & Hunt, D.F. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 101, 9528–9533 (2004).
Udeshi, N.D., Compton, P.D., Shabanowitz, J., Hunt, D.F. & Rose, K.L. Methods for analyzing peptides and proteins on a chromatographic timescale by electron-transfer dissociation mass spectrometry. Nat. Protoc. 3, 1709–1717 (2008).
Schroeder, M.J., Shabanowitz, J., Schwartz, J.C., Hunt, D.F. & Coon, J.J. A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal. Chem. 76, 3590–3598 (2004).
Earley, L. et al. Front-end electron transfer dissociation: a new ionization source. Anal. Chem. 85, 8385–8390 (2013).
Wiesner, J., Premsler, T. & Sickmann, A. Application of electron transfer dissociation (ETD) for the analysis of posttranslational modifications. Proteomics 8, 4466–4483 (2008).
Mommen, G.P.M. et al. Expanding the detectable HLA peptide repertoire using electron-transfer/higher-energy collision dissociation (EThcD). Proc. Natl Acad. Sci. USA 111, 4507–4512 (2014).
Martin, S.E., Shabanowitz, J., Hunt, D.F. & Marto, J.A. Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 72, 4266–4274 (2000).
Prilliman, K. et al. Large-scale production of class I bound peptides: assigning a signature to HLA-B*1501. Immunogenetics 45, 379–385 (1997).
Hawkins, O.E. et al. Identification of breast cancer peptide epitopes presented by HLA-A*0201. J. Proteome Res. 7, 1445–1457 (2008).
Purcell, A. Isolation and characterization of naturally processed MHC-bound peptides from the surface of antigen-presenting cells in HPLC of Peptides and Proteins: Methods and Protocols (ed. Aguilar, M.-I.) 251, 291–306 (Springer New York, 2004).
Geer, L.Y. et al. Open mass spectrometry search algorithm. J. Proteome Res. 3, 958–964 (2004).
Acknowledgements
This work was supported by the US National Institutes of Health with grants AI 033993 and GM O37537 (to D.F.H.).
Author information
Authors and Affiliations
Contributions
J.G.A., J.S. and D.F.H. designed the studies, and J.G.A. and P.D.T. executed them. S.A.P. and M.C. immunopurified HLA-associated peptides from colorectal cancer tumors that were resected by S.T.W. from consenting patients. A.M.P. and W.H.H. transfected FHIOSE cells with sHLA-A*0201 and isolated associated peptides. D.L.B. developed programs that were used during data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Full MS and MS2 Spectra demonstrating phosphopeptide sequencing using both CAD and ETD fragmentation.
A zoomed-in full MS spectrum showing the (M+3H)3+ peptide precursor ion isolated using a 3 m/z window for ETD and CAD fragmentation. The CAD MS2 spectrum demonstrates that the neutral loss of phosphoric acid from the precursor ion results in an MS2 spectrum that is difficult to interpret. The ETD MS2 spectrum of the same precursor enables the sequencing of this phosphopeptide because it preserves the labile post translational modification. Even though the CAD MS2 spectrum contains few sequence informative ions, it can be used to identify which precursors in the data are likely phosphopeptides because it contains the characteristic neutral loss of 98 Daltons. The “CAD Neutral Loss Finder” program is used to identify these spectra so they can be manually interpreted.
Supplementary Figure 2 Diagram of pressure bomb setup and microcapillary column fabrication.
Schematic depicting the pressure bomb setup used for handling fused silica microcapillary columns. The pressure cell is connected to a helium gas tank though a 3 way valve. Inside the pressure cell is a vial that contains a slurry of the desired packing material. A Kasil® 1624 fritted fused silica microcapillary column is inserted in the Teflon ferrule in the Swagelock brass fitting with the open end of the column in the packing slurry. The top of the pressure cell is screwed on, and the column is packed against the Kasil® 1624 frit when the inside chamber is pressurized with He gas.
Supplementary Figure 3 CAD MS2 spectra of a synthetic phosphopeptide compared to the same phosphopeptide found experimentally used for sequence validation.
Example MS and MS2 data are shown to illustrate our method of manual validation. A) Experimental data for the HLA-associated phosphopeptide RTLsHISEA. A lowercase “s” indicates that the serine is phosphorylated in the peptide sequence. The b- and y-ions for the esterified form of RTLsHISEA are shown with the expected mass shifts for esterification (14 Da) and phosphorylation (80 Da). A zoomed-in total ion current chromatogram (TIC) and a base peak chromatogram (Base Peak) from an enrichment experiment are displayed. Extracted ion chromatograms for the peptide precursor ion (561.2712 ± 5 ppm) and the MS2 of this precursor are also displayed. A zoomed-in Full MS spectrum depicts the (M+2H)2+ precursor ion at 561.2727 m/z, and the CAD MS2 spectrum of the precursor shows the relative abundances of the peptide fragment ions produced. B) An MS2 spectrum of the experimentally observed RTLsHISEA peptide labeled with the b- and y-ions produced from CAD fragmentation. Neutral losses of water from the b- and y-ions are labeled as “°” in the MS2 spectra. B-ions are labeled in blue, and y-ions are labeled in red. C) An MS2 spectrum of the synthetic RTLsHISEA peptide labeled with the b- and y-ions produced from CAD fragmentation. The synthetic peptide was used to validate that the correct sequence was assigned to the experimentally observed phosphopeptide.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 564 kb)
Rights and permissions
About this article
Cite this article
Abelin, J., Trantham, P., Penny, S. et al. Complementary IMAC enrichment methods for HLA-associated phosphopeptide identification by mass spectrometry. Nat Protoc 10, 1308–1318 (2015). https://doi.org/10.1038/nprot.2015.086
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.086
This article is cited by
-
Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire
Nature Reviews Clinical Oncology (2020)
-
Mass spectrometry–based identification of MHC-bound peptides for immunopeptidomics
Nature Protocols (2019)
-
Murine xenograft bioreactors for human immunopeptidome discovery
Scientific Reports (2019)
-
Phytic acid functionalized Fe3O4 nanoparticles loaded with Ti(IV) ions for phosphopeptide enrichment in mass spectrometric analysis
Microchimica Acta (2019)
-
Recent applications of metal–organic frameworks in matrix-assisted laser desorption/ionization mass spectrometry
Analytical and Bioanalytical Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.