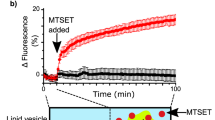
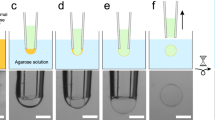
Abstract
We describe a protocol for forming an artificial lipid bilayer by contacting nanoliter aqueous droplets in an oil solution in the presence of phospholipids. A lipid monolayer forms at each oil-water interface, and when two such monolayers touch, a bilayer is created. Droplet interface bilayers (DIBs) are a simple way to generate stable bilayers suitable for single-channel electrophysiology and optical imaging from a wide variety of preparations, ranging from purified proteins to reconstituted eukaryotic cell membrane fragments. Examples include purified proteins from the α-hemolysin pore from Staphylococcus aureus, the anthrax toxin pore and the 1.2-MDa mouse mechanosensitive channel MmPiezo1. Ion channels and ionotropic receptors can also be reconstituted from membrane fragments without further purification. We describe two approaches for forming DIBs. In one approach, a lipid bilayer is created between two aqueous droplets submerged in oil. In the other approach, a membrane is formed between an aqueous droplet and an agarose hydrogel, which allows imaging in addition to electrical recordings. The protocol takes <30 min, including droplet generation, monolayer assembly and bilayer formation. In addition to the main protocol, we also describe the preparation of Ag/AgCl electrodes and sample preparation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sackmann, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Dzieciol, A.J. & Mann, S. Designs for life: protocell models in the laboratory. Chem. Soc. Rev. 41, 79–85 (2012).
Fendler, J.H. Atomic and molecular clusters in membrane mimetic chemistry. Chem. Rev. 87, 877–899 (1987).
Decher, G. Membranes and more. Membrane-mimetic approach to advanced materials. Adv. Mater. 7, 421 (1995).
Chang, T. Artificial cells in medicine and biotechnology. Appl. Biochem. Biotechnol. 10, 5–24 (1984).
Thutupalli, S., Herminghaus, S. & Seemannc, R. Bilayer membranes in micro-fluidics: from gel emulsions to soft functional devices. Soft Matter 7, 1312–1320 (2011).
Castell, O.K., Berridge, J. & Wallace, M.I. Quantification of membrane protein inhibition by optical ion flux in a droplet interface bilayer array. Angew. Chem. Int. Ed. Engl. 51, 3134–3138 (2012).
Syeda, R., Holden, M.A., Hwang, W.L. & Bayley, H. Screening blockers against a potassium channel with a droplet interface bilayer array. J. Am. Chem. Soc. 130, 15543–15548 (2008).
Castellana, E.T. & Cremer, P.S. Solid supported lipid bilayers: from biophysical studies to sensor design. Surf. Sci. Rep. 61, 429–444 (2006).
Tanaka, M. & Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 437, 656–663 (2005).
Tamm, L.K. & McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 47, 105–113 (1985).
Dewa, T. et al. Lateral organization of a membrane protein in a supported binary lipid domain: direct observation of the organization of bacterial light-harvesting complex 2 by total internal reflection fluorescence microscopy. Langmuir 22, 5412–5418 (2006).
Atanasov, V. et al. Membrane on a chip: a functional tethered lipid bilayer membrane on silicon oxide surfaces. Biophys. J. 89, 1780–1788 (2005).
Rawle, R.J., van Lengerich, B., Chung, M., Bendix, P.M. & Boxer, S.G. Vesicle fusion observed by content transfer across a tethered lipid bilayer. Biophys. J. 101, L37–L39.
Mueller, P., Rudin, D.O., Ti Tien, H. & Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194, 979–980 (1962).
Montal, M. & Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 69, 3561–3566 (1972).
Cheng, H.T. & London, E. Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys. J. 100, 2671–2678 (2011).
Richmond, D.L. et al. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc. Natl. Acad. Sci. USA 108, 9431–9436 (2011).
Szoka Jr., F. & Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 9, 467–508 (1980).
Bayley, H. et al. Droplet interface bilayers. Mol. Biosyst. 4, 1191–1208 (2008).
Holden, M.A., Needham, D. & Bayley, H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 129, 8650–8655 (2007).
Maglia, G. et al. Droplet networks with incorporated protein diodes show collective properties. Nat. Nanotechnol. 4, 437–440 (2009).
Hwang, W.L., Chen, M., Cronin, B., Holden, M.A. & Bayley, H. Asymmetric droplet interface bilayers. J. Am. Chem. Soc. 130, 5878–5879 (2008).
Gross, L.C., Castell, O.K. & Wallace, M.I. Dynamic and reversible control of 2D membrane protein concentration in a droplet interface bilayer. Nano Lett. 11, 3324–3328 (2011).
Heron, A.J., Thompson, J.R., Cronin, B., Bayley, H. & Wallace, M.I. Simultaneous measurement of ionic current and fluorescence from single protein pores. J. Am. Chem. Soc. 131, 1652–1653 (2009).
Heron, A.J., Thompson, J.R., Mason, A.E. & Wallace, M.I. Direct detection of membrane channels from gels using water-in-oil droplet bilayers. J. Am. Chem. Soc. 129, 16042–16047 (2007).
Leptihn, S., Thompson, J.R., Ellory, J.C., Tucker, S.J. & Wallace, M.I. In vitro reconstitution of eukaryotic ion channels using droplet interface bilayers. J. Am. Chem. Soc. 133, 9370–9375 (2011).
Funakoshi, K., Suzuki, H. & Takeuchi, S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal. Chem. 78, 8169–8174 (2006).
Malmstadt, N., Nash, M.A., Purnell, R.F. & Schmidt, J.J. Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett. 6, 1961–1965 (2006).
Jeon, T.J., Malmstadt, N., Poulos, J.L. & Schmidt, J.J. Black lipid membranes stabilized through substrate conjugation to a hydrogel. Biointerphases 3, FA96 (2008).
Poulos, J.L., Portonovo, S.A., Bang, H. & Schmidt, J.J. Automatable lipid bilayer formation and ion channel measurement using sessile droplets. J. Phys. Condens. Matter. 22, 454105 (2010).
Poulos, J.L. et al. Ion channel and toxin measurement using a high-throughput lipid membrane platform. Biosens. Bioelectron. 24, 1806–1810 (2009).
Takinoue, M. & Takeuchi, S. Droplet microfluidics for the study of artificial cells. Anal. Bioanal. Chem. 400, 1705–1716 (2011).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Sarles, S.A. & Leo, D.J. Physical encapsulation of droplet interface bilayers for durable, portable biomolecular networks. Lab. Chip 10, 710–717 (2010).
Jeon, T.J., Poulos, J.L. & Schmidt, J.J. Long-term storable and shippable lipid bilayer membrane platform. Lab. Chip 8, 1742–1744 (2008).
Stanley, C.E. et al. A microfluidic approach for high-throughput droplet interface bilayer (DIB) formation. Chem. Commun. 46, 1620–1622 (2010).
Jason, L.P., Wyatt, C.N., Tae-Joon, J., Chang-Jin, C.J.K. & Jacob, J.S. Electrowetting on dielectric-based microfluidics for integrated lipid bilayer formation and measurement. Appl. Phys. Lett. 95, 013706 (2009).
Hu, P.C., Li, S. & Malmstadt, N. Microfluidic fabrication of asymmetric giant lipid vesicles. ACS Appl. Mater. Interfaces 3, 1434–1440 (2011).
Dixit, S.S., Kim, H., Vasilyev, A., Eid, A. & Faris, G.W. Light-driven formation and rupture of droplet bilayers. Langmuir 26, 6193–6200 (2010).
Punnamaraju, S., You, H. & Steckl, A.J. Triggered release of molecules across droplet interface bilayer lipid membranes using photopolymerizable lipids. Langmuir 28, 7657–7664 (2012).
Portonovo, S.A. & Schmidt, J. Masking apertures enabling automation and solution exchange in sessile droplet lipid bilayers. Biomed. Microdevices 14, 187–191 (2012).
Punnamaraju, S. & Steckl, A.J. Voltage control of droplet interface bilayer lipid membrane dimensions. Langmuir 27, 618–626 (2011).
Gross, L.C., Heron, A.J., Baca, S.C. & Wallace, M.I. Determining membrane capacitance by dynamic control of droplet interface bilayer area. Langmuir 27, 14335–14342 (2011).
Huang, J., Lein, M., Gunderson, C. & Holden, M.A. Direct quantitation of peptide-mediated protein transport across a droplet-interface bilayer. J. Am. Chem. Soc. 133, 15818–15821 (2011).
Fischer, A., Holden, M.A., Pentelute, B.L. & Collier, R.J. Ultrasensitive detection of protein translocated through toxin pores in droplet-interface bilayers. Proc. Natl. Acad. Sci USA 108, 16577–16581 (2011).
Thompson, J.R., Heron, A.J., Santoso, Y. & Wallace, M.I. Enhanced stability and fluidity in droplet on hydrogel bilayers for measuring membrane protein diffusion. Nano Lett. 7, 3875–3878 (2007).
Xu, J., Sigworth, F.J. & LaVan, D.A. Synthetic protocells to mimic and test cell function. Adv. Mater. 22, 120–127 (2010).
Harriss, L.M., Cronin, B., Thompson, J.R. & Wallace, M.I. Imaging multiple conductance states in an alamethicin pore. J. Am. Chem. Soc. 133, 14507–14509 (2011).
Tien, H.T. & Ottova, A.L. The lipid bilayer concept and its experimental realization: from soap bubbles, kitchen sink, to bilayer lipid membranes. J. Memb. Sci. 189, 83–117 (2001).
Haverkamp, R. Membrane magic and the magic of membranes: planar lipid bilayers (BLMs) and their applications. Biotechnol. Adv. 22, 311–312 (2004).
Blodgett, K.B. Monomolecular films of fatty acids on glass. J. Am. Chem. Soc. 56, 495–495 (1934).
Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids.1. J. Am. Chem. Soc. 39, 1848–1906 (1917).
Petty, M.C. Langmuir-Blodgett Films (Cambridge University Press, 1996).
Morales-Penningston, N.F. et al. GUV preparation and imaging: minimizing artifacts. Biochim. Biophys. Acta. 1798, 1324–1332 (2010).
Montes, L.R., Alonso, A., Goni, F.M. & Bagatolli, L.A. Giant unilamellar vesicles electroformed from native membranes and organic lipid mixtures under physiological conditions. Biophys. J. 93, 3548–3554 (2007).
Zagnoni, M. Miniaturised technologies for the development of artificial lipid bilayer systems. Lab. Chip 12, 1026–1039 (2012).
Suzuki, H. & Takeuchi, S. Microtechnologies for membrane protein studies. Anal. Bioanal. Chem. 391, 2695–2702 (2008).
Aghdaei, S., Sandison, M.E., Zagnoni, M., Green, N.G. & Morgan, H. Formation of artificial lipid bilayers using droplet dielectrophoresis. Lab. Chip 8, 1617–1620 (2008).
Malmstadt, N., Hoffman, A.S. & Stayton, P.S. 'Smart' mobile affinity matrix for microfluidic immunoassays. Lab. Chip 4, 412–415 (2004).
White, S.H. Ion Channel Reconstitution (ed. Milller, C.) (Plenum Press, 1986).
Rigaud, J.L. & Levy, D. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 372, 65–86 (2003).
Paternostre, M.T., Roux, M. & Rigaud, J.L. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by triton X-100, octyl glucoside, and sodium cholate. Biochemistry 27, 2668–2677 (1988).
Schimerlik, M.I. Overview of membrane protein solubilization. Curr. Protoc. Neurosci. published online; http://dx.doi.org/10.1002/0471142301.ns0509s02 (1 May 2001).
Hanke, W. & Schule, W.R. Planar Lipid Bilayers: Methods and Applications (Academic Press, 1993).
Acknowledgements
We thank H. Bayley for his comments. This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC). M.H. is funded by the US National Science Foundation (NSF; CAREER award no. 1253565). M.I.W. is funded by the European Research Council (ERC). B.C. is an Engineering and Physical Sciences Research Council (EPSRC) Life Sciences Interface (LSI) Postdoctoral Fellow.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. M.H. and E.-H.L. conducted the experiments on droplet-droplet DIBs. L.C.M.G. and S.L. conducted the experiments on droplet-hydrogel DIBs. B.C., S.L. and M.I.W. prepared the figures. J.R.T. helped develop the protocol. S.L., D.P.M., O.K.C., M.H. and M.I.W. wrote the main paper. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Leptihn, S., Castell, O., Cronin, B. et al. Constructing droplet interface bilayers from the contact of aqueous droplets in oil. Nat Protoc 8, 1048–1057 (2013). https://doi.org/10.1038/nprot.2013.061
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.061
This article is cited by
-
Transmembrane coupling of liquid-like protein condensates
Nature Communications (2023)
-
Spatiotemporal stop-and-go dynamics of the mitochondrial TOM core complex correlates with channel activity
Communications Biology (2022)
-
Building programmable multicompartment artificial cells incorporating remotely activated protein channels using microfluidics and acoustic levitation
Nature Communications (2022)
-
Molecular substructure of the liquid-ordered phase formed by sphingomyelin and cholesterol: sphingomyelin clusters forming nano-subdomains are a characteristic feature
Biophysical Reviews (2022)
-
Constructing ion channels from water-soluble α-helical barrels
Nature Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.