Abstract
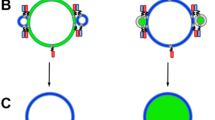
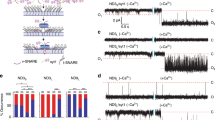
This protocol describes an assay that uses suspended nanomembranes called nanodiscs to analyze fusion events. A nanodisc is a lipid bilayer wrapped by membrane scaffold proteins. Fluorescent lipids and a protein that is part of a fusion machinery, VAMP2 in the example detailed herein, are included in the nanodiscs. Upon fusion of a nanodisc with a nonfluorescent liposome containing cognate proteins (for instance, the VAMP2 cognate syntaxin1/SNAP-25 complex), the fluorescent lipids are dispersed in the liposome and the increase in fluorescence, initially quenched in the nanodisc, is monitored on a plate reader. Because the scaffold proteins restrain pore expansion, the fusion pore eventually reseals. A reducing agent, such as dithionite, which can quench the fluorescence of accessible lipids, can then be used to determine the number of fusion events. A fluorescence-based approach can also be used to monitor the release of encapsulated cargo. From data on the total cargo release and the number of the much faster lipid-mixing events, the researcher may determine the amount of cargo released per fusion event. This assay requires 3 d for preparation and 4 h for data acquisition and analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bonifacino, J.S. & Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 116, 153–166 (2004).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Wickner, W. & Schekman, R. Membrane fusion. Nat. Struct. Mol. Biol. 15, 658–664 (2008).
Jahn, R. & Scheller, R.H. SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006).
Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698 (2008).
Friedlander-Shani, L. & Podbilewicz, B. Heterochronic control of AFF-1-mediated cell-to-cell fusion in C. elegans. Adv. Exp. Med. Biol. 713, 5–11 (2011).
Youle, R.J. & van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Fernandez-Peruchena, C., Navas, S., Montes, M.A. & Alvarez de Toledo, G. Fusion pore regulation of transmitter release. Brain Res. Brain Res. Rev. 49, 406–415 (2005).
Struck, D.K., Hoekstra, D. & Pagano, R.E. Use of resonance energy-transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 (1981).
McNew, J.A. et al. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol. 150, 105–117 (2000).
van den Bogaart, G. et al. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 17, 358–364 (2010).
Stengel, G., Zahn, R. & Hook, F. DNA-induced programmable fusion of phospholipid vesicles. J. Am. Chem. Soc. 129, 9584–9585 (2007).
Scott, B.L. et al. Liposome fusion assay to monitor intracellular membrane fusion machines. Methods Enzymol. 372, 274–300 (2003).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Shen, J., Tareste, D.C., Paumet, F., Rothman, J.E. & Melia, T.J. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 (2007).
Hernandez, J.M. et al. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science 336, 1581–1584 (2012).
Smith, E.A. & Weisshaar, J.C. Docking, not fusion, as the rate-limiting step in a SNARE-driven vesicle fusion assay. Biophys. J. 100, 2141–2150 (2011).
Ji, H. et al. Protein determinants of SNARE-mediated lipid mixing. Biophys. J. 99, 553–560 (2010).
Langosch, D. et al. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J. Mol. Biol. 311, 709–721 (2001).
Shi, L. et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335, 1355–1359 (2012).
Ritchie, T.K. et al. Chapter 11—Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009).
Frauenfeld, J. et al. Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat. Struct. Mol. Biol. 18, 614–621 (2011).
Katayama, H. et al. Three-dimensional structure of the anthrax toxin pore inserted into lipid nanodiscs and lipid vesicles. Proc. Natl. Acad. Sci. USA 107, 3453–3457 (2010).
Brewer, K.D., Li, W., Horne, B.E. & Rizo, J. Reluctance to membrane binding enables accessibility of the synaptobrevin SNARE motif for SNARE complex formation. Proc. Natl. Acad. Sci. USA 108, 12723–12728 (2011).
Imai, M., Mizuno, T. & Kawasaki, K. Membrane fusion by single influenza hemagglutinin trimers. Kinetic evidence from image analysis of hemagglutinin-reconstituted vesicles. J. Biol. Chem. 281, 12729–12735 (2006).
Bayburt, T.H., Grinkova, Y.V. & Sligar, S.G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 (2002).
Koppaka, V. & Axelsen, P.H. Lipoprotein A-I structure. Trends Cardiovasc. Med. 9, 192–195 (1999).
Nath, A., Atkins, W.M. & Sligar, S.G. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 46, 2059–2069 (2007).
Bayburt, T.H. & Sligar, S.G. Membrane protein assembly into Nanodiscs. FEBS Lett. 584, 1721–1727 (2009).
Raschle, T. et al. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J. Am. Chem. Soc. 131, 17777–17779 (2009).
Boldog, T., Grimme, S., Li, M., Sligar, S.G. & Hazelbauer, G.L. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc. Natl. Acad. Sci. USA 103, 11509–11514 (2006).
McNew, J.A., Weber, T., Engelman, D.M., Sollner, T.H. & Rothman, J.E. The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol. Cell 4, 415–421 (1999).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Schaub, J.R., Lu, X., Doneske, B., Shin, Y.K. & McNew, J.A. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat. Struct. Mol. Biol. 13, 748–750 (2006).
Melia, T.J. et al. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 158, 929–940 (2002).
Acknowledgements
This work was supported by a ANR-09-Blanc-0129 grant to F.P., US National Institutes of Health (NIH) grant DK027044 to J.E.R. and a Partner University Funds exchange grant between the Yale University and Ecole Normale Supérieure laboratories. We thank T. Melia for many helpful discussions.
Author information
Authors and Affiliations
Contributions
L.S., J.E.R. and F.P. designed the research. L.S., Y.J.W. and K.H. optimized the detailed protocol. Q.-T. Shen performed the electron microscopy. L.S., K.H. and F.P. analyzed the experiments. L.S., K.H., Y.J.W. and F.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Shi, L., Howan, K., Shen, QT. et al. Preparation and characterization of SNARE-containing nanodiscs and direct study of cargo release through fusion pores. Nat Protoc 8, 935–948 (2013). https://doi.org/10.1038/nprot.2013.048
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.048
This article is cited by
-
Actual fusion efficiency in the lipid mixing assay - Comparison between nanodiscs and liposomes
Scientific Reports (2017)
-
Exocytotic fusion pores are composed of both lipids and proteins
Nature Structural & Molecular Biology (2016)
-
Nanodisc-cell fusion: control of fusion pore nucleation and lifetimes by SNARE protein transmembrane domains
Scientific Reports (2016)
-
Cryo-EM structure of SNAP-SNARE assembly in 20S particle
Cell Research (2015)
-
Progress in measuring biophysical properties of membrane proteins with AFM single-molecule force spectroscopy
Chinese Science Bulletin (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.