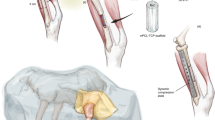
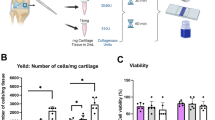
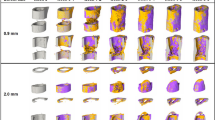
Abstract
Animal models that are reliably reproducible, appropriate analogs to the clinical condition they are used to investigate, and that offer minimal morbidity and periprocedural mortality to the subject, are the keystone to the preclinical development of translational technologies. For bone tissue engineering, a number of small animal models exist. Here we describe the protocol for one such model, the rat calvarial defect. This versatile model allows for evaluation of biomaterials and bone tissue engineering approaches within a reproducible, non-load-bearing orthotopic site. Crucial steps for ensuring appropriate experimental control and troubleshooting tips learned through extensive experience with this model are provided. The surgical procedure itself takes ∼30 min to complete, with ∼2 h of perioperative care, and tissue collection is generally performed 4−12 weeks postoperatively. Several analytical techniques are presented, which evaluate the cellular and extracellular matrix components, functionality and mineralization, including histological, mechanical and radiographic methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Giannoudis, P.V. & Atkins, R. Management of long-bone non-unions. Injury 38, S1–S2 (2007).
Giannoudis, P.V. et al. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J. Bone Joint Surg. Br. 82, 655–658 (2000).
Schmitz, J.P. & Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 205, 299–308 (1986).
Cancedda, R., Giannoni, P. & Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28, 4240–4250 (2007).
Reichert, J.C. et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng. Part B Rev. 16, 93–104 (2010).
Bodde, E.W.H., Spauwen, P.H.M., Mikos, A.G. & Jansen, J.A. Closing capacity of segmental radius defects in rabbits. J. Biomed. Mater. Res. A 85, 206–217 (2008).
Pearce, A.I., Richards, R.G., Milz, S., Schneider, E. & Pearce, S.G. Animal models for implant biomaterial research in bone: a review. Eur. Cells Mater. 13, 1–10 (2007).
Einhorn, T.A. Clinically applied models of bone regeneration in tissue engineering research. Clin. Orthop. Relat. Res. S59–S67 (1999).
Einhorn, T., Lane, J., Burstein, A., Kopman, C. & Vigorita, V. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J. Bone Joint Surg. Am. 66, 274–279 (1984).
Wang, J.C. et al. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J. Bone Joint Surg. Am. 85, 905–911 (2003).
Henslee, A.M. et al. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 7, 3627–3637 (2011).
Kempen, D.H.R. et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 29, 3245–3252 (2008).
Kempen, D.H.R. et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30, 2816–2825 (2009).
Kempen, D.H.R. et al. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J. Control Release 134, 169–176 (2009).
Freeman, E. & Turnbull, R.S. The value of osseous coagulum as a graft material. J. Periodont. Res. 8, 229–236 (1973).
Takagi, K. & Urist, M.R. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann. Surg. 196, 100–109 (1982).
Ferland, C.E., Laverty, S., Beaudry, F. & Vachon, P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol. Biochem. Behav. 97, 603–610 (2011).
Kim, K.S. et al. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells. Biomaterials 31, 1104–1113 (2010).
Muschler, G.F., Raut, V.P., Patterson, T.E., Wenke, J.C. & Hollinger, J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 16, 123–145 (2010).
Hobar, P.C., Masson, J.A., Wilson, R. & Zerwekh, J. The importance of the dura in craniofacial surgery. Plast. Reconstr. Surg. 98, 217–225 (1996).
Hobar, P.C., Schreiber, J.S., McCarthy, J.G. & Thomas, P.A. The role of the dura in cranial bone regeneration in the immature animal. Plast. Reconstr. Surg. 92, 405–410 (1993).
Cooper, G.M. et al. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 125, 1685–1692 (2010).
Kretlow, J.D. et al. Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical size rat cranial defects. Tissue Eng. Part A 16, 3555–3568 (2010).
Patel, Z.S. et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 (2008).
Bodde, E.W.H. et al. The kinetic and biological activity of different loaded rhBMP-2 calcium phosphate cement implants in rats. J. Biomed. Mater. Res. A 87, 780–791 (2008).
Ruhé, P.Q. et al. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J. Biomed. Mater. Res. 74, 533–544 (2005).
Cooper, G.M. et al. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng. Part A 16, 1749–1759 (2010).
Chesmel, K.D., Branger, J., Wertheim, H. & Scarborough, N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J. Oral Maxillofac. Surg. 56, 857–863; discussion 864–855 (1998).
Schmökel, H.G. et al. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J. Orthop. Res. 22, 376–381 (2004).
Schmökel, H.G. et al. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 89, 253–262 (2005).
Blum, J., Barry, M., Mikos, A.G. & Jansen, J.A. In vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarial defect model. Hum. Gene Ther. 14, 1689–1701 (2003).
Chew, S.A. et al. Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model. Tissue Eng. Part A 17, 751–763 (2011).
Kasper, F.K. et al. Evaluation of bone regeneration by DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in a critical-sized calvarial defect. J. Biomed. Mater. Res. B Appl. Biomater. 78, 335–342 (2006).
Huang, Y.-C., Simmons, C., Kaigler, D., Rice, K.G. & Mooney, D.J. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4). Gene Therapy 12, 418–426 (2005).
Kim, S.-S. & Kim, B.-S. Comparison of osteogenic potential between apatite-coated poly(lactide-co-glycolide)/hydroxyapatite particulates and Bio-Oss. Dent. Mater. J. 27, 368–375 (2008).
Dhert, W.J. et al. A finite element analysis of the push-out test: influence of test conditions. J. Biomed. Mater. Res. B Appl. Biomater. 26, 119–130 (1992).
Knothe, U.R., Dolejs, S., Miller, R.M. & Knothe Tate, M.L. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect healing. J. Biomech. 43, 2728–2737 (2010).
Krause, W.R., Bradbury, D.W., Kelly, J.E. & Lunceford, E.M. Temperature elevations in orthopaedic cutting operations. J. Biomech. 15, 267–275 (1982).
Mabbutt, L.W., Kokich, V.G., Moffett, B.C. & Loeser, J.D. Subtotal neonatal calvariectomy. A radiographic and histological evaluation of calvarial and sutural redevelopment in rabbits. J. Neurosurg. 51, 691–696 (1979).
Persson, K.M., Roy, W.A., Persing, J.A., Rodeheaver, G.T. & Winn, H.R. Craniofacial growth following experimental craniosynostosis and craniectomy in rabbits. J. Neurosurg. 50, 187–197 (1979).
Reid, C.A., McCarthy, J.G. & Kolber, A.B. A study of regeneration in parietal bone defects in rabbits. Plast. Reconstr. Surg. 67, 591–596 (1981).
Mountziaris, P.M. & Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 14, 179–186 (2008).
Mountziaris, P.M., Spicer, P.P., Kasper, F.K. & Mikos, A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B Rev. 17, 393–402 (2011).
American Veterinary Medical Association (AVMA). AVMA guidelines on euthanasia. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (AVMA, 2007).
National Research Council. Guide for the Care and Use of Laboratory Animals (The National Academies Press, 1996).
Pelker, R.R., Friedlaender, G.E., Markham, T.C., Panjabi, M.M. & Moen, C.J. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J. Orthop. Res. 1, 405–411 (1983).
Kallai, I. et al. Microcomputed tomography–based structural analysis of various bone tissue regeneration models. Nat. Protoc. 6, 105–110 (2011).
Young, S. et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng. Part A 15, 2347–2362 (2009).
Duvall, C.L., Taylor, W.R., Weiss, D. & Guldberg, R.E. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 287, H302–H310 (2004).
An, Y.H. & Martin, K.L. Handbook of Histology Methods for Bone and Cartilage (Humana Press, 2003).
Acknowledgements
We acknowledge support by the US National Institutes of Health (grant nos. R21 AR56076, R01 DE015164, R01 AR42639 and R01 DE017441) for research toward the development of biomaterials for bone tissue engineering. In addition, we thank the veterinary and husbandry staff, as well as the members of the Institutional Animal Care and Use Committee at Rice University, who have had a crucial role in the development of this protocol.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. J.D.K. and P.P.S. developed the mechanical testing methodology and wrote the manuscript. J.D.K., P.P.S., S.Y., J.A.J. and F.K.K. performed or aided in animal surgeries, microCT assessment and histological assessment. S.Y. developed the microCT and angiogenesis evaluation methodologies. J.A.J. and A.G.M. supervised all methodology development and experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Spicer, P., Kretlow, J., Young, S. et al. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 7, 1918–1929 (2012). https://doi.org/10.1038/nprot.2012.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.113
This article is cited by
-
Genipin versus Ferric Chloride cross-linked unmodified Gum Arabic/Chitosan/nano-Hydroxyapatite nanocomposite hydrogels as potential scaffolds for bone regeneration
Scientific Reports (2023)
-
Chondrocytes supplemented to bone graft-containing scaffolds expedite cranial defect repair
Scientific Reports (2023)
-
Integrating bioprinting, cell therapies and drug delivery towards in vivo regeneration of cartilage, bone and osteochondral tissue
Drug Delivery and Translational Research (2023)
-
Role of animal models in biomedical research: a review
Laboratory Animal Research (2022)
-
MiR-1224-5p modulates osteogenesis by coordinating osteoblast/osteoclast differentiation via the Rap1 signaling target ADCY2
Experimental & Molecular Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.