Abstract
Spatiotemporal retroviral gene transfer into specific somatic mammalian cells using the avian RCAS (replication-competent avian sarcoma-leukosis virus long terminal repeat with splice acceptor)/tumor virus A (TVA) system is a versatile tool for performing lineage tracing and gene function analysis in vivo. RCAS retroviruses carrying the subgroup A envelope transduce only genetically engineered mammalian cells that express the cognate avian retroviral receptor TVA. The RCAS/TVA gene delivery system has been successfully used in various different mouse TVA-expression models. This protocol contains a detailed description of the production of high-titer RCAS retroviruses in chicken fibroblasts and the transduction of proliferating TVA-positive somatic mammalian cells in vivo. By taking advantage of the combination of the RCAS/TVA with the 'universal' Cre/loxP system, the protocol can be used in nearly every proliferating cell type in vivo. The protocol takes 4 weeks from transfection of chicken fibroblasts, which act as the host cells for viral production, to the transduction of TVA-transgenic mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brugge, J.S. & Erikson, R.L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature 269, 346–348 (1977).
Weiss, R.A. & Vogt, P.K. 100 years of Rous sarcoma virus. J. Exp. Med. 208, 2351–2355 (2011).
Federspiel, M.J. & Hughes, S.H. Retroviral gene delivery. Methods Cell Biol. 52, 179–214 (1997).
Hughes, S.H., Greenhouse, J.J., Petropoulos, C.J. & Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 61, 3004–3012 (1987).
Greenhouse, J.J., Petropoulos, C.J., Crittenden, L.B. & Hughes, S.H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J. Virol. 62, 4809–4812 (1988).
Petropoulos, C.J. & Hughes, S.H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J. Virol. 65, 3728–3737 (1991).
Boerkoel, C.F. et al. A new defective retroviral vector system based on the Bryan strain of Rous sarcoma virus. Virology 195, 669–679 (1993).
Bates, P., Young, J.A. & Varmus, H.E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74, 1043–1051 (1993).
Young, J.A., Bates, P. & Varmus, H.E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 67, 1811–1816 (1993).
Himly, M. et al. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248, 295–304 (1998).
Schaefer-Klein, J. et al. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology 248, 305–311 (1998).
Lewis, B.C., Chinnasamy, N., Morgan, R.A. & Varmus, H.E. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector. J. Virol. 75, 9339–9344 (2001).
El Maarouf, A., Petridis, A.K. & Rutishauser, U. Use of polysialic acid in repair of the central nervous system. Proc. Natl. Acad. Sci. USA 103, 16989–16994 (2006).
Petropoulos, C.J., Payne, W., Salter, D.W. & Hughes, S.H. Appropriate in vivo expression of a muscle-specific promoter by using avian retroviral vectors for gene transfer [corrected]. J. Virol. 66, 3391–3397 (1992).
Bromberg-White, J.L. et al. Delivery of short hairpin RNA sequences by using a replication-competent avian retroviral vector. J. Virol. 78, 4914–4916 (2004).
Holland, E.C., Hively, W.P., Gallo, V. & Varmus, H.E. Modeling mutations in the G1 arrest pathway in human gliomas: overexpression of CDK4 but not loss of INK4a-ARF induces hyperploidy in cultured mouse astrocytes. Genes Dev. 12, 3644–3649 (1998).
Orsulic, S. et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1, 53–62 (2002).
Frese, K.K. & Tuveson, D.A. Maximizing mouse cancer models. Nat. Rev. Cancer 7, 645–658 (2007).
Sauer, B. Inducible gene targeting in mice using the Cre/lox system. Methods 14, 381–392 (1998).
Xu, Q. & Anderson, S.A. Mapping lineage using BAC-Cre reporter lines. Curr. Protoc. Neurosci. Chapter 1, Unit 1 19 (2010).
von Burstin, J. et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 137, 361–371, 371 eC61–365 (2009).
Eser, S. et al. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proc. Natl. Acad. Sci. USA 108, 9945–9950 (2011).
Olive, K.P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Tuveson, D. & Hanahan, D. Translational medicine: cancer lessons from mice to humans. Nature 471, 316–317 (2011).
Jonkers, J. & Berns, A. Conditional mouse models of sporadic cancer. Nat. Rev. Cancer 2, 251–265 (2002).
Kinzler, K.W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Singer, O. & Verma, I.M. Applications of lentiviral vectors for shRNA delivery and transgenesis. Curr. Gene Ther. 8, 483–488 (2008).
Seidler, B. et al. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc. Natl. Acad. Sci. USA 105, 10137–10142 (2008).
Du, Y.C., Lewis, B.C., Hanahan, D. & Varmus, H. Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion. PLoS Biol. 5, e276 (2007).
Orsulic, S. An RCAS-TVA-based approach to designer mouse models. Mamm. Genome 13, 543–547 (2002).
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Fisher, G.H. et al. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene 18, 5253–5260 (1999).
Pao, W., Klimstra, D.S., Fisher, G.H. & Varmus, H.E. Use of avian retroviral vectors to introduce transcriptional regulators into mammalian cells for analyses of tumor maintenance. Proc. Natl. Acad. Sci. USA 100, 8764–8769 (2003).
Holmen, S.L. & Williams, B.O. Essential role for Ras signaling in glioblastoma maintenance. Cancer Res. 65, 8250–8255 (2005).
Federspiel, M.J. et al. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc. Natl. Acad. Sci. USA 91, 11241–11245 (1994).
Brown, K.N. et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science 334, 480–486 (2011).
Holland, E.C. & Varmus, H.E. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc. Natl. Acad. Sci. USA 95, 1218–1223 (1998).
Lewis, B.C., Klimstra, D.S. & Varmus, H.E. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 17, 3127–3138 (2003).
Lewis, B.C. et al. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol. Cell Biol. 25, 1228–1237 (2005).
Du, Z. et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc. Natl. Acad. Sci. USA 103, 17396–17401 (2006).
Beier, K.T., Samson, M.E., Matsuda, T. & Cepko, C.L. Conditional expression of the TVA receptor allows clonal analysis of descendents from Cre-expressing progenitor cells. Dev. Biol. 353, 309–320 (2011).
Li, L. et al. An in vivo model to study osteogenic gene regulation: targeting an avian retroviral receptor (TVA) to bone with the bone sialoprotein (BSP) promoter. J. Bone Miner. Res. 20, 1403–1413 (2005).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Feil, R. Conditional somatic mutagenesis in the mouse using site-specific recombinases. Handb. Exp. Pharmacol. 178, 3–28 (2007).
Wickersham, I.R., Sullivan, H.A. & Seung, H.S. Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nat. Protoc. 5, 595–606 (2010).
DuPage, M., Dooley, A.L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Tiscornia, G., Singer, O. & Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 1, 241–245 (2006).
Murphy, G.J. & Leavitt, A.D. A model for studying megakaryocyte development and biology. Proc. Natl. Acad. Sci. USA 96, 3065–3070 (1999).
Dunn, K.J., Williams, B.O., Li, Y. & Pavan, W.J. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc. Natl. Acad. Sci. USA 97, 10050–10055 (2000).
Vervoort, V.S. et al. A novel Flk1-TVA transgenic mouse model for gene delivery to angiogenic vasculature. Transgenic Res. 17, 403–415 (2008).
Nakhai, H. et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development 134, 1151–1160 (2007).
O'Gorman, S., Dagenais, N.A., Qian, M. & Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 94, 14602–14607 (1997).
Mayr, U. et al. RCAS-mediated retroviral gene delivery: a versatile tool for the study of gene function in a mouse model of pancreatic cancer. Hum. Gene Ther. 19, 896–906 (2008).
Hafner, C. et al. Multiple oncogenic mutations and clonal relationship in spatially distinct benign human epidermal tumors. Proc. Natl. Acad. Sci. USA 107, 20780–20785 (2010).
Loftus, S.K. et al. Generation of RCAS vectors useful for functional genomic analyses. DNA Res. 8, 221–226 (2001).
Federspiel, M.J. et al. Expression of transduced genes in mice generated by infecting blastocysts with avian leukosis virus-based retroviral vectors. Proc. Natl. Acad. Sci. USA 93, 4931–4936 (1996).
Lindberg, N. et al. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene 28, 2266–2275 (2009).
Gaur, M., Murphy, G.J., deSauvage, F.J. & Leavitt, A.D. Characterization of Mpl mutants using primary megakaryocyte-lineage cells from Mpl−/− mice: a new system for Mpl structure-function studies. Blood 97, 1653–1661 (2001).
Bu, W. et al. Keratin 6a marks mammary bipotential progenitor cells that can give rise to a unique tumor model resembling human normal-like breast cancer. Oncogene 30, 4399–4409 (2011).
Morton, J.P. et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc. Natl. Acad. Sci. USA 104, 5103–5108 (2007).
Holland, E.C., Hively, W.P., DePinho, R.A. & Varmus, H.E. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 12, 3675–3685 (1998).
Schuller, U. et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134 (2008).
Hou, L. et al. Complementation of melanocyte development in SOX10 mutant neural crest using lineage-directed gene transfer. Dev. Dyn. 229, 54–62 (2004).
Murphy, G.J. et al. Manipulation of mouse hematopoietic progenitors by specific retroviral infection. J. Biol. Chem. 278, 43556–43563 (2003).
Montaner, S. et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3, 23–36 (2003).
Acknowledgements
We would like to thank H. Nakhai (Technical University Munich) and S. O'Gorman (Case Western Reserve University) for providing transgenic animals. We are grateful to N. Proudfoot (University of Oxford), P. Bates (University of Pennsylvania), S. Orsulic (Harvard Medical School) and S.H. Hughes (US National Cancer Institute) for providing vectors. This work was supported by funding from Deutsche Krebshilfe (no. 108985 to D.S.). Research was conducted in compliance with the European guidelines for the care and use of laboratory animals and approved by the local authorities.
Author information
Authors and Affiliations
Contributions
A.v.W., B.S. and D.S. performed research and developed the protocol; A.v.W., B.S., R.M.S., G.S. and D.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
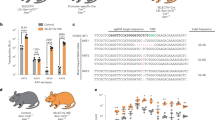
Scheme of the wild type Rosa26 (R26) locus and the targeted R26 locus with the integrated lox-stop-lox (LSL) silenced Tva-IRES-LacZnls cassette. Copyright (2008) National Academy of Sciences, U.S.A. Reproduced with permission from Seidler, B. et al. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc Natl Acad Sci U S A 105, 10137-10142 (2008). (A) Upper panel: Scheme of the R26 wild type locus. Primer binding sites for genotyping of the R26 wild type locus are depicted. Lower panel: Targeted Rosa26 locus. Black arrowheads flanking the transcriptional stop cassette represent loxP sites. Primer binding sites are indicated by arrows. SA: Splice acceptor. ATG: ATG start codon of TVA. (B-E): Genotyping PCR. (B) PCR analysis of the wild type (WT) and the targeted LSL-R26Tva-lacZ allele. (C) PCR for detection of TVA cDNA or genomic DNA in the mouse, regardless of the TVA strain. (D) PCR to test integrity of the lox-stop-lox (LSL) cassette before Cre mediated recombination. (E) PCR to test Cre mediated deletion of the LSL cassette. Sizes of wild-type and mutant PCR products are indicated. (DOC 113 kb)
Supplementary Method 1
Genotyping protocol for LSL-R26Tva-lacZ/+ , Ptf1aCre/+ and Prm-Cre animals (DOC 57 kb)
Rights and permissions
About this article
Cite this article
von Werder, A., Seidler, B., Schmid, R. et al. Production of avian retroviruses and tissue-specific somatic retroviral gene transfer in vivo using the RCAS/TVA system. Nat Protoc 7, 1167–1183 (2012). https://doi.org/10.1038/nprot.2012.060
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.060
This article is cited by
-
New α- and SIN γ-retrovectors for safe transduction and specific transgene expression in pancreatic β cell lines
BMC Biotechnology (2019)
-
Somatic genome editing with the RCAS-TVA-CRISPR-Cas9 system for precision tumor modeling
Nature Communications (2018)
-
Functional requirement of a wild-type allele for mutant IDH1 to suppress anchorage-independent growth through redox homeostasis
Acta Neuropathologica (2018)
-
Continuous cell supply from Krt7-expressing hematopoietic stem cells during native hematopoiesis revealed by targeted in vivo gene transfer method
Scientific Reports (2017)
-
Somatic cell transfer of c-Myc and Bcl-2 induces large-cell anaplastic medulloblastomas in mice
Journal of Neuro-Oncology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.