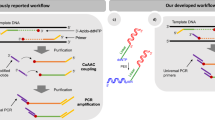
Abstract
Linear amplification for deep sequencing (LADS) is an amplification method that produces representative libraries for Illumina next-generation sequencing within 2 d. The method relies on attaching two different sequencing adapters to blunt-end repaired and A-tailed DNA fragments, wherein one of the adapters is extended with the sequence for the T7 RNA polymerase promoter. Ligated and size-selected DNA fragments are transcribed in vitro with high RNA yields. Subsequent cDNA synthesis is initiated from a primer complementary to the first adapter, ensuring that the library will only contain full-length fragments with two distinct adapters. Contrary to the severely biased representation of AT- or GC-rich fragments in standard PCR-amplified libraries, the sequence coverage in T7-amplified libraries is indistinguishable from that of nonamplified libraries. Moreover, in contrast to amplification-free methods, LADS can generate sequencing libraries from a few nanograms of DNA, which is essential for all applications in which the starting material is limited.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Metzker, M.L. Sequencing technologies—the next generation. Nat. Rev. Genet. 11, 31–46 (2010).
Wilhelm, B.T., Marguerat, S., Goodhead, I. & Bahler, J. Defining transcribed regions using RNA-seq. Nat. Protoc. 5, 255–266 (2010).
Bock, C. et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat. Biotechnol. 28, 1106–1114 (2010).
Brinkman, A.B. et al. Whole-genome DNA methylation profiling using MethylCap-seq. Methods 52, 232–236 (2010).
Park, P.J. ChIP-seq: advantages and challenges of a maturing technology. Nat. Rev. Genet. 10, 669–680 (2009).
Akkers, R.C. et al. A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev. Cell 17, 425–434 (2009).
Vermeulen, M. et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142, 967–980 (2010).
Tang, F. et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 5, 516–535 (2010).
Mamanova, L. et al. FRT-seq: amplification-free, strand-specific transcriptome sequencing. Nat. Methods 7, 130–132 (2010).
Armour, C.D. et al. Digital transcriptome profiling using selective hexamer priming for cDNA synthesis. Nat. Methods 6, 647–649 (2009).
Cloonan, N. et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat. Methods 5, 613–619 (2008).
Core, L.J., Waterfall, J.J. & Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Ingolia, N.T. Genome-wide translational profiling by ribosome footprinting. Methods Enzymol. 470, 119–142 (2010).
Kozarewa, I. et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat. Methods 6, 291–295 (2009).
Quail, M.A. et al. A large genome center's improvements to the Illumina sequencing system. Nat. Methods 5, 1005–1010 (2008).
Meyer, M., Stenzel, U. & Hofreiter, M. Parallel tagged sequencing on the 454 platform. Nat. Protoc. 3, 267–278 (2008).
Bentley, D.R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456, 53–59 (2008).
Bartfai, R. et al. H2A.Z demarcates intergenic regions of the Plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog. 6, e1001223 (2010).
Goren, A. et al. Chromatin profiling by directly sequencing small quantities of immunoprecipitated DNA. Nat. Methods 7, 47–49 (2010).
Hillier, L.W. et al. Whole-genome sequencing and variant discovery in C. elegans. Nat. Methods 5, 183–188 (2008).
Linnarsson, S. Recent advances in DNA sequencing methods—general principles of sample preparation. Exp. Cell Res. 316, 1339–1343 (2010).
Gardner, M.J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 (2002).
Glockner, G. et al. Sequence and analysis of chromosome 2 of Dictyostelium discoideum. Nature 418, 79–85 (2002).
Perelygina, L. et al. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 77, 6167–6177 (2003).
Liu, C.L., Schreiber, S.L. & Bernstein, B.E. Development and validation of a T7 based linear amplification for genomic DNA. BMC Genomics 4, 19 (2003).
Salcedo-Amaya, A.M. et al. Dynamic histone H3 epigenome marking during the intraerythrocytic cycle of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 106, 9655–9660 (2009).
van Bakel, H. et al. Improved genome-wide localization by ChIP-chip using double-round T7 RNA polymerase-based amplification. Nucleic Acids Res. 36, e21 (2008).
Cole, S.T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Eid, J. et al. Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138 (2009).
Fire, A. & Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 92, 4641–4645 (1995).
Liu, D., Daubendiek, S.L., Zillman, M.A., Ryan, K. & Kool, E.T. Rolling circle DNA synthesis: small circular oligonucleotides as efficient templates for DNA polymerases. J. Am. Chem. Soc. 118, 1587–1594 (1996).
Dean, F.B. et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl. Acad. Sci. USA 99, 5261–5266 (2002).
Atherton, R.A. et al. Whole genome sequencing of enriched chloroplast DNA using the Illumina GAII platform. Plant Methods 6, 22 (2010).
Jeng, S.T., Gardner, J.F. & Gumport, R.I. Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J. Biol. Chem. 265, 3823–3830 (1990).
Krieg, P.A. Improved synthesis of full-length RNA probe at reduced incubation temperatures. Nucleic Acids Res. 18, 6463 (1990).
O'Neill, L.P. & Turner, B.M. Immunoprecipitation of native chromatin: NChIP. Methods 31, 76–82 (2003).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Mortazavi, A., Williams, B.A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Dohm, J.C., Lottaz, C., Borodina, T. & Himmelbauer, H. Substantial biases in ultra-short read data sets from high-throughput DNA sequencing. Nucleic Acids Res. 36, e105 (2008).
Acknowledgements
The development of this method has been financially supported by the Netherlands Organisation for Scientific Research (ZonMw/NGI Horizon 93511023; NWO-Toptalent 021.001.011) and by the European Commission (EVIMalaR; ATLAS EU-FP7_221952). We thank E. Janssen-Megens for technical assistance, advice and operation of the GAII machine and D. van Soolingen and A. Schurch for providing Mycobacterium tuberculosis genomic DNA. Furthermore, we thank A. Salcedo-Amaya and A. Brinkman for useful discussions during the development of LADS and for critical reading of the manuscript, and we are grateful for the advice and help of all our colleagues in the molecular biology department.
Author information
Authors and Affiliations
Contributions
R.B. developed the concept of LADS. W.A.M.H. and R.B. established and fine-tuned the protocol. K.-J.F. provided bioinformatic support. H.G.S. supervised the project. W.A.M.H., R.B. and H.G.S. prepared and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1 | Schematic representation of standard and amplification-free library preparation for Illumina sequencing.
(a) In the standard Illumina protocol (www.illumina.com) end-polished and A-tailed DNA fragments are ligated with a "sparrow tail" adapter containing a short complementary region and two different sequencing primers (S1, S2) on the forward and reverse strands. After size-selection, ligated fragments are subject to 12 (or even 18) cycles of PCR amplification to increase copy number. The "extended" PCR primers are also used to incorporate the sequences that facilitate hybridization to the oligonucleotides (P5, P7) attached to the surface of the flowcell; (b) In the amplification-free protocol (ref. 14) the "sparrow tail" adapters already contain the sequence used for capturing on the surface of the flowcell. Therefore, after ligation and size selection, they can be directly used for sequencing. (TIFF 581 kb)
Supplementary Figure 2 | LADS preserves GC-rich regions of the M. tuberculosis genome.
(a-c) Screenshots of the shotgun sequencing data obtained by different library preparation methods from M. tuberculosis. 40 or 400 ng sonicated genomic DNA from SH1:NLA000700874 strain was used to prepare sequencing libraries by standard PCR-based method (40ng starting material with 12 cycles of amplification), amplification-free method (400ng starting material) or LADS (40ng starting material). After sequencing on the Illumina GAII platform, 36bp sequence reads were mapped against the reference genome (H37Rv). Coverage plots were generated from equal number (∼21 million) of unique and randomly placed non-unique reads by counting the number of tags in every 10bp window of the genome. Ratio-plots display the sequencing data obtained after amplification (PCR or T7) over the amplification-free control in log2 scale (150bp moving window smoothening has been applied). (a) genome-wide view, (b-c) “zoom-in” to regions with extreme GC-content. (TIFF 1912 kb)
Supplementary Figure 3 | Decrosslinking introduces sequence bias in NGS libraries.
Formaldehyde cross-linked, fragmented Plasmodium falciparum chromatin was decrosslinked for 4 hours at 60°C or overnight at 45°C in the presence of 0.2 or 0.5M NaCl. After purification, DNA was used for Illumina library preparation using linear T7 amplification and analyzed by qPCR for amplicons with different AT-content. (TIFF 612 kb)
Rights and permissions
About this article
Cite this article
Hoeijmakers, W., Bártfai, R., Françoijs, KJ. et al. Linear amplification for deep sequencing. Nat Protoc 6, 1026–1036 (2011). https://doi.org/10.1038/nprot.2011.345
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.345
This article is cited by
-
Toward efficient and high-fidelity metagenomic data from sub-nanogram DNA: evaluation of library preparation and decontamination methods
BMC Biology (2022)
-
Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning
Nature Biomedical Engineering (2021)
-
An epigenetic map of malaria parasite development from host to vector
Scientific Reports (2020)
-
Evaluation of bias induced by viral enrichment and random amplification protocols in metagenomic surveys of saliva DNA viruses
Microbiome (2018)
-
Characterization of chromatin accessibility with a transposome hypersensitive sites sequencing (THS-seq) assay
Genome Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.