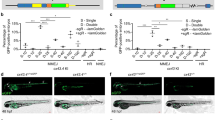
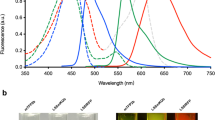
Abstract
Zebrafish are a useful vertebrate model for the study of development, behavior, disease and cancer. A major advantage of zebrafish is that large numbers of animals can be economically used for experimentation; however, high-throughput methods for imaging live adult zebrafish had not been developed. Here, we describe protocols for building a light-emitting diode (LED) fluorescence macroscope and for using it to simultaneously image up to 30 adult animals that transgenically express a fluorescent protein, are transplanted with fluorescently labeled tumor cells or are tagged with fluorescent elastomers. These protocols show that the LED fluorescence macroscope is capable of distinguishing five fluorescent proteins and can image unanesthetized swimming adult zebrafish in multiple fluorescent channels simultaneously. The macroscope can be built and used for imaging within 1 day, whereas creating fluorescently labeled adult zebrafish requires 1 hour to several months, depending on the method chosen. The LED fluorescence macroscope provides a low-cost, high-throughput method to rapidly screen adult fluorescent zebrafish and it will be useful for imaging transgenic animals, screening for tumor engraftment, and tagging individual fish for long-term analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
de Jong, J.L.O. & Zon, L.I. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu. Rev. Genet. 39, 481–501 (2005).
Drummond, I.A. Kidney development and disease in the zebrafish. J. Am. Soc. Nephrol. 16, 299–304 (2005).
Amatruda, J.F., Patton, E.E. & Kwang, W.J. Genetic models of cancer in zebrafish. Int. Rev. Cell Mol. Biol. 271, 1–34 (2008).
Stoletov, K. & Klemke, R. Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 (2008).
Taylor, A.M. & Zon, L.I. Zebrafish tumor assays: the state of transplantation. Zebrafish 6, 339–346 (2009).
Meeker, N.D. & Trede, N.S. Immunology and zebrafish: spawning new models of human disease. Dev. Comp. Immunol. 32, 745–757 (2008).
Gerlai, R., Lahav, M., Guo, S. & Rosenthal, A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 67, 773–782 (2000).
Darland, T. & Dowling, J.E. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc. Natl Acad. Sci. USA 98, 11691–11696 (2001).
Panula, P. et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 40, 46–57 (2010).
Speedie, N. & Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 188, 168–177 (2008).
Smith, A.C.H. et al. High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 115, 3296–3303 (2010).
Ma, R., Taruttis, A., Ntziachristos, V. & Razansky, D. Multispectral optoacoustic tomography (MSOT) scanner for whole-body small animal imaging. Opt. Express 17, 21414–21426 (2009).
Kabli, S., Alia, A., Spaink, H.P., Verbeek, F.J. & De Groot, H.J.M. Magnetic resonance microscopy of the adult zebrafish. Zebrafish 3, 431–439 (2006).
Goessling, W., North, T.E. & Zon, L.I. Ultrasound biomicroscopy permits in vivo characterization of zebrafish liver tumors. Nat. Methods 4, 551–553 (2007).
Goldsmith, R., Closs, G. & steen, H. Evaluation of visible implant elastomer as a method for individual marking of small perch and common bully. J. Fish Biol. 63, 631–636 (2003).
Josephon, D.C., Robinson, J.M., Weidel, B.C. & Kraft, C.E. Long-term retention and visibility of visible implant elastomer tags in brook trout. North Am. J. Fish. Manag. 28, 1758–1761 (2008).
Simmons, A., Padsalgikar, A.D., Ferris, L.M. & Poole-Warren, L.A. Biostability and biological performance of a PDMS-based polyurethane for controlled drug release. Biomaterials 29, 2987–2995 (2008).
Mizgireuv, I.V. & Revskoy, S. Generation of clonal zebrafish lines and transplantable hepatic tumors. Nat. Protoc. 5, 383–394 (2010).
Mizgireuv, I.V. & Revskoy, S.Y. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 66, 3120–3125 (2006).
Langenau, D.M. et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 299, 887–890 (2003).
Langenau, D.M. et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 21, 1382–1395 (2007).
Langenau, D.M. et al. Co-injection strategies to modify radiation sensitivity and tumor initiation in transgenic zebrafish. Oncogene 27, 4242–4248 (2008).
Rosen, J.N. et al. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 25 (published online doi:10.3791/1115 09 March 2009).
Berghmans, S. et al. Making waves in cancer research: new models in the zebrafish. BioTechniques 39, 227–237 (2005).
Acknowledgements
J.S.B. is supported by National Institutes of Health (NIH) grant 5T32CA09216-26. C.D.S. is supported by the Dev and Linda Gupta Fund. D.M.L. is supported by NIH grants K01 AR055619-01A1 and 3 K01 AR055619-03S1, the Alex's Lemonade Stand Foundation, the Sarcoma Foundation of America, the Hollis Brownstein Research Grant from the Leukemia Research Foundation and by a grant from the Harvard Stem Cell Institute. We thank Northwest Marine Technology for expert technical advice and for providing visible implant elastomers for our pilot experiments.
Author information
Authors and Affiliations
Contributions
S.L., A.R.R., M.S.I. and C.D.S. conducted the experiments; J.S.B. and D.M.L. conducted the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Basic construction of the inverted LED fluorescence macroscope. Measured photographs of the Inverted LED fluorescence macroscope highlighting the basic construction of the external shell. Right side view (A), front/top down view (B), top view (C), bottom view (D, the final 1/4” plywood cover is not shown),Top view after lights fixtures have been added (E), and bottom view after light fixtures have been added (F). Red arrows in D denote the attachments for securing the bottom of the macroscope to the wood frame. Blue arrow in F shows where the 1” hole should be drilled that receives wires to be connected to the switch box. (JPG 942 kb)
Supplementary Figure 2
Attaching the switch box and lights to the inverted LED fluorescence macroscope. Measured photograph of the inverted LED fluorescence macroscope highlighting attachment of the switch box, lights, and top of the macroscope. The switch box is attached to the macroscope by glue (A). Wooden 5/8” square dowel side supports are shown in B,C. Front/top view of the macroscope imaged with the wood top cover (D). Arrows in D denote placement of screws to support the bottom of themacroscope. Top view of macroscope (E,F), with the front side noted. (JPG 918 kb)
Supplementary Figure 3
Wiring the inverted LED fluorescence macroscope. The correct wiring technique is shown in A-D. Wire connections are shown for the black wires only (E). Circuit diagram of how to wire the macroscope (F). (JPG 748 kb)
Supplementary Figure 4
Preparing the electric switch box for the inverted LED fluorescence macroscope. The two tabs shown in A are removed using a saw (A, red lines) and the plastic tab is removed on the switch box (B, asterisk) to receive wires. (JPG 599 kb)
Supplementary Figure 5
Wiring the switches for the inverted LED fluorescence macroscope. The wires are connected to the switch (A), then the switches are attached to the blue switch box (B), and the switch plate cover is installed C). (JPG 371 kb)
Supplementary Figure 6
Setting up the camera for the inverted LED fluorescence macroscope. The camera is connected to a mounting bracket and wood insert (A-E). A ring of PVC is glued to the camera to house the optical filter (D). Multiple cameras can be attached to the wood insert for multispectral imaging. (F,G). The wood insert with cameras is placed within the inverted macroscope (H). (JPG 722 kb)
Supplementary Figure 7
Photographs of the upright LED fluorescence macroscope. Upright LED fluorescence macroscope imaged from above (A), below (B), the front (C), the back (D), the left side (E), and the right side (F). (JPG 838 kb)
Supplementary Figure 8
Measurements of the upright LED fluorescence macroscope. The upright LED fluorescence macroscope imaged from above (A), below (B), the front (C), the back (D), the left side (E), and the right side (F). Lengths of PVC tubing used in each connection are shown (A-D). The green box in panel A shows the corner connection, which is depicted in detail in Supplementary Figure 9. (JPG 1077 kb)
Supplementary Figure 9
Creating corner connectors for the upright LED fluorescence macroscope. PVC tubing and connectors used in creating the upright LED fluorescence macroscope (A, some connectors not shown), and parts used to create the corner connector (B-D). (JPG 687 kb)
Supplementary Figure 10
Wiring the upright LED fluorescence macroscope. Lights are attached to a T-type PVC connector that has a center threaded 1/2” PVC opening. The light is screwed into place and wires are connected through the inside of the PVC tubing (A). Once completed, the lights can be attached to the macroscope (B). A waterproof switch plate cover is used(C), and end cap with hole drilled through allows the wire to be plugged in to the wall outlet (D). (JPG 808 kb)
Supplementary Figure 11
Wiring diagram for the upright LED fluorescence macroscope. The negative wire is denoted by red and positive by black. All wiring is contained within the PVC piping, which is water-tight. (JPG 277 kb)
Supplementary Figure 12
Setting up the camera for the LED fluorescence macroscope. To attach the camera, holes are drilled in the center PVC pipe (A,B). Cameras are glued onto a mounting bracket as in Supplementary Figure 6, then the camera is attached to the PVC pipe using a wing nut (C,D). (JPG 698 kb)
Supplementary Movie 1
Multispectral video of swimming transgenic zebrafish. mylz-mCherry and mylz-GFP fish were imaged using a blue light with 640/35nm and 535/45nm filters. The capture rate is 60ms. (MOV 9697 kb)
Supplementary Manual 1
Instructions for building the inverted and upright macroscopes. (DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Blackburn, J., Liu, S., Raimondi, A. et al. High-throughput imaging of adult fluorescent zebrafish with an LED fluorescence macroscope. Nat Protoc 6, 229–241 (2011). https://doi.org/10.1038/nprot.2010.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.170
This article is cited by
-
“Sea”-ing Is Believing: In Vivo Imaging of Hematopoietic Stem Cells and Cancer Using Zebrafish
Current Stem Cell Reports (2017)
-
A fresh look at zebrafish from the perspective of cancer research
Journal of Experimental & Clinical Cancer Research (2015)
-
Notch signaling expands a pre-malignant pool of T-cell acute lymphoblastic leukemia clones without affecting leukemia-propagating cell frequency
Leukemia (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.