Abstract
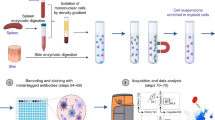
We describe two modular protocols for immunostaining and multiparameter flow cytometric analysis of major human antigen-presenting cells (APCs; e.g., dendritic cells, monocytes and B lymphocytes) in minimally manipulated whole blood samples. Simultaneous detection of up to eight colors is enabled by careful selection and testing of cell-subset-defining monoclonal antibodies (anchor markers) in the appropriate fluorochrome combinations, in order to show the quantification of surface expression levels of molecules involved in chemotaxis (e.g., CX3CR1 and CCR2), adhesion (e.g., CD11b and CD62L), antigen presentation (e.g., CD83, CD86 and CD209) and immune regulation (e.g., CD101) on circulating APCs. Each immunostaining reaction requires as little as 50–100 μl of peripheral whole blood and no density-gradient separation, and the entire procedure from preparation of reagents to flow cytometry can be completed in <5 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ziegler-Heitbrock, H.W.L. Definition of human blood monocytes. J. Leukoc. Biol. 67, 603–606 (2000).
Grage-Griebenow, E., Flad, H.D. & Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 (2001).
Steinman, R.M. & Nussenzweig, M.C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA 99, 351–358 (2002).
Al-Daccak, R., Mooney, N. & Charron, D. MHC class II signaling in antigen-presenting cells. Curr. Opin. Immunol. 16, 108–113 (2004).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Tacke, F. et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. 203, 583–597 (2006).
Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483 (2006).
Davis, M.M. A prescription for human immunology. Immunity 29, 835–838 (2008).
Hayday, A.C. & Peakman, M. The habitual, diverse and surmountable obstacles to human immunology research. Nat. Immunol. 9, 575–580 (2008).
Baumgarth, N. & Roederer, M. A practical approach to multicolor flow cytometry for immunophenotyping. J. Immunol. Methods 243, 77–97 (2000).
Perfetto, S.P., Chattopadhyay, P.K. & Roederer, M. Seventeen-colour flow cytometry: unravelling the immune system. Nat. Rev. Immunol. 4, 648–655 (2004).
Willmann, K. & Dunne, J.F. A flow cytometric immune function assay for human peripheral blood dendritic cells. J. Leukoc. Biol. 67, 536–544 (2000).
Seder, R.A., Darrah, P.A. & Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008).
Dendrou, C.A. et al. Gene-to-cell-specific protein phenotype for the autoimmune locus IL2RA using fresh blood from a genotype-selectable bioresource. Nat. Genet. 41, 1011–1015 (2009).
Tamul, K.R., Schmitz, J.L., Kane, K. & Folds, J.D. Comparison of the effects of Ficoll-Hypaque separation and whole blood lysis on results of immunophenotypic analysis of blood and bone marrow samples from patients with hematologic malignancies. Clin. Diagn. Lab Immunol. 2, 337–342 (1995).
Jansen, K. et al. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J. Immunol. Methods 336, 183–192 (2008).
Perfetto, S.P., Ambrozak, D., Nguyen, R., Chattopadhyay, P. & Roederer, M. Quality assurance for polychromatic flow cytometry. Nat. Protoc. 1, 1522–1530 (2006).
Dendrou, C.A. et al. Fluorescence intensity normalisation: correcting for time effects in large-scale flow cytometric analysis. Adv. Bioinformatics 2009 doi:10.1155/2009/476106.
Passlick, B., Flieger, D. & Ziegler-Heitbrock, H.W.L. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 (1989).
Geissmann, F., Jung, S. & Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Fingerle, G. et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 82, 3170–3176 (1993).
Kawanaka, N. et al. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis. Rheum. 46, 2578–2586 (2002).
Belge, K.U. et al. The proinflammatory CD14+CD16+DR. monocytes are a major source of TNF. J. Immunol. 168, 3536–3542 (2002).
Spits, H., Couwenberg, F., Bakker, A.Q., Weijer, K. & Uittenbogaart, C.H. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192, 1775–1784 (2000).
Liu, Y.J., Kanzler, H., Soumelis, V. & Gilliet, M. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2, 585–589 (2001).
Liu, Y.J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 (2005).
Münz, C., Steinman, R.M. & Fujii, S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 202, 203–207 (2005).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Fogg, D. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Dzionek, A. et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037–6046 (2000).
MacDonald, K.P. et al. Characterization of human blood dendritic cell subsets. Blood 100, 4512–4520 (2002).
Bonasio, R. & von Andrian, U.H. Generation, migration and function of circulating dendritic cells. Curr. Opin. Immunol. 18, 503–511 (2006).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 194, 1823–1834 (2001).
Radbruch, A. Immunofluorescence: basic considerations. In Flow Cytometry and Cell Sorting 2nd edn 2. Chapter 3 (Springer-Verlag Berlin, Heidelberg, Germany, 2000).
Herzenberg, L.A., Tung, J., Moore, W.A., Herzenberg, L.A. & Parks, D.R. Interpreting flow cytometry data: a guide for the perplexed. Nat. Immunol. 7, 681–685 (2006).
Roederer, M. Spectral compensation for flow cytometry: visualization artefacts, limitations, and caveats. Cytometry 45, 194–205 (2001).
Shapiro, H.M. Parameters and probes. In Practical Flow Cytometry 4th edn. Chapter 7 (John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2003).
Maecker, H.T., Frey, T., Nomura, L.E. & Trotter, J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 62A, 169–173 (2004).
Maecker, H.T. & Trotter, J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A 69A, 1037–1042 (2006).
Kawai, K. et al. CD11b-mediated migratory property of peripheral blood B cells. J. Allergy Clin. Immunol. 116, 192–197 (2005).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 (2006).
Arnold, L.W., Pennell, C.A., McCray, S.K. & Clarke, S.H. Development of B-1 cells: segregation of phosphatidylcholine-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 179, 1585–1595 (1994).
Nestle, F.O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Summers, K.L. et al. Reduced IFN-alpha secretion by blood dendritic cells in human diabetes. Clin. Immunol. 121, 81–89 (2006).
Peng, R., Li, Y., Brezner, K., Litherland, S. & Clare-Salzler, M.J. Abnormal peripheral blood dendritic cell populations in type 1 diabetes. Ann. NY Acad. Sci. 1005, 222–225 (2003).
Allen, J.S. et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 58, 138–145 (2009).
Ochando, J.C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7, 652–662 (2006).
Shao, L., Jacobs, A.R., Johnson, V.V. & Mayer, L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174, 7539–7547 (2005).
Allez, M., Brimnes, J., Dotan, I. & Mayer, L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology 123, 1516–1526 (2002).
Bouloc, A., Bagot, M., Delaire, S., Bensussan, A. & Boumsell, L. Triggering CD101 molecule on human cutaneous dendritic cells inhibits T cell proliferation via IL-10 production. Eur. J. Immunol. 30, 3132–3139 (2000).
Ashtekar, A.R. & Saha, B. Poly′s plea: membership to the club of APCs. Trends Immunol. 24, 485–490 (2003).
Rivas, A. et al. V7, a novel leukocyte surface protein that participates in T cell activation. I. Tissue distribution and functional studies. J. Immunol. 154, 4423–4433 (1995).
Pyne, S. et al. Automated high-dimensional flow cytometric data analysis. Proc. Natl. Acad. Sci. USA 106, 8519–8524 (2009).
Acknowledgements
We thank the Wellcome Trust, the Juvenile Diabetes Research Foundation (JDRF) International and the National Institute for Health Research Cambridge Biomedical Research Centre for funding. The Cambridge Institute for Medical Research is a recipient of the Wellcome Trust Strategic Award (079895). E.F. was funded by the JDRF and the Prince Philip Graduate Exhibition from the Cambridge Overseas Trust. L.S.W. is a Wellcome Trust Principal Research Fellow.
Author information
Authors and Affiliations
Contributions
E.F. contributed to the conception, design, performance and analysis of the protocols and wrote the manuscript. L.E. contributed to the configuration and establishment of the flow cytometer in the laboratory and to the writing of the manuscript. J.A.T. participated in the conception of the protocols and the writing of the manuscript. L.S.W. contributed to the conception, design and analysis of the protocols and to the writing of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Fung, E., Esposito, L., Todd, J. et al. Multiplexed immunophenotyping of human antigen-presenting cells in whole blood by polychromatic flow cytometry. Nat Protoc 5, 357–370 (2010). https://doi.org/10.1038/nprot.2009.246
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.246
This article is cited by
-
Qualifying antibodies for image-based immune profiling and multiplexed tissue imaging
Nature Protocols (2019)
-
Tumor microenvironmental plasmacytoid dendritic cells contribute to breast cancer lymph node metastasis via CXCR4/SDF-1 axis
Breast Cancer Research and Treatment (2019)
-
A multi‐laboratory comparison of blood dendritic cell populations
Clinical & Translational Immunology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.