Abstract
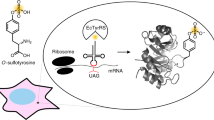
Tyrosine sulfation is an important post-translational modification that occurs in higher eukaryotes and is involved in cell–cell communication, viral entry and adhesion. We describe a protocol for the heterologous expression of selectively tyrosine-sulfated proteins in Escherichia coli through the use of an expanded genetic code that co-translationally inserts sulfotyrosine in response to the amber nonsense codon, TAG. The components required for this process, an orthogonal aminoacyl-tRNA synthetase specific for sulfotyrosine and its cognate orthogonal tRNA that recognizes the amber codon, are encoded on the plasmid pSUPAR6-L3-3SY, and their use, along with a simple chemical synthesis of sulfotyrosine, are outlined in this protocol. Specifically, the gene for a protein of interest is mutated such that the codon corresponding to the desired location of tyrosine sulfate is TAG. Co-transformation of an expression vector containing this gene and pSUPAR6-L3-3SY into an appropriate E. coli strain allows the overexpression of the site-specifically sulfated protein with high efficiency and fidelity. The resulting protein contains tyrosine sulfate at any location specified by a TAG codon, making this method significantly simpler and more versatile than competing methods such as in vitro enzymatic sulfation, chemical sulfation and peptide synthesis. Once the proper expression vectors are cloned, our protocol should allow the production of the desired sulfated proteins in <1 week.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Moore, K.L. The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 278, 24243–24246 (2003).
Seibert, C. & Sakmar, T.P. Toward a framework for sulfoproteomics: synthesis and characterization of sulfotyrosine-containing peptides. Biopolymers 90, 459–477 (2008).
Kehoe, J.W. & Bertozzi, C.R. Tyrosine sulfation: a modulator of extracellular protein–protein interactions. Chem. Biol. 7, R57–R61 (2000).
Preobrazhensky, A.A. et al. Monocyte chemotactic protein-1 receptor CCR2B is a glycoprotein that has tyrosine sulfation in a conserved extracellular N-terminal region. J. Immunol. 165, 5295–5303 (2000).
Somers, W.S., Tang, J., Shaw, G.D. & Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 103, 467–479 (2000).
Veldkamp, C.T. et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci. Signal. 1, ra4 (2008).
Veldkamp, C.T., Seibert, C., Peterson, F.C., Sakmar, T.P. & Volkman, B.F. Recognition of a CXCR4 sulfotyrosine by the chemokine stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12). J. Mol. Biol. 359, 1400–1409 (2006).
Farzan, M. et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96, 667–676 (1999).
Dorfman, T., Moore, M.J., Guth, A.C., Choe, H. & Farzan, M. A tyrosine-sulfated peptide derived from the heavy-chain CDR3 region of an HIV-1-neutralizing antibody binds gp120 and inhibits HIV-1 infection. J. Biol. Chem. 281, 28529–28535 (2006).
Farzan, M. et al. The role of post-translational modifications of the CXCR4 amino terminus in stromal-derived factor 1 alpha association and HIV-1 entry. J. Biol. Chem. 277, 29484–29489 (2002).
Choe, H. et al. Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC). Mol. Microbiol. 55, 1413–1422 (2005).
Hans, D. et al. Mapping binding residues in the Plasmodium vivax domain that binds Duffy antigen during red cell invasion. Mol. Microbiol. 55, 1423–1434 (2005).
Liu, C.C. & Schultz, P.G. Recombinant expression of selectively sulfated proteins in Escherichia coli . Nat. Biotechnol. 24, 1436–1440 (2006).
Liu, C.C., Brustad, E., Liu, W. & Schultz, P.G. Crystal structure of a biosynthetic sulfo-hirudin complexed to thrombin. J. Am. Chem. Soc. 129, 10648–10649 (2007).
Liu, C.C. et al. Protein evolution with an expanded genetic code. Proc. Natl. Acad. Sci. USA 105, 17688–17693 (2008).
Cellitti, S.E. et al. In vivo incorporation of unnatural amino acids to probe structure, dynamics, and ligand binding in a large protein by nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 130, 9268–9281 (2008).
Nemeth-Cawley, J.F., Karnik, S. & Rouse, J.C. Analysis of sulfated peptides using positive electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 36, 1301–1311 (2001).
Penke, B. & Nyerges, L. Solid-phase synthesis of porcine cholecystokinin-33 in a new resin via FMOC-strategy. Pept. Res. 4, 289–295 (1991).
Acknowledgements
C.C.L. thanks the Fannie and John Hertz Foundation and the National Science Foundation for predoctoral fellowships. This research was supported by the US National Institutes of Health (GM62159).
Author information
Authors and Affiliations
Contributions
C.C.L., S.E.C., B.H.G. and P.G.S. designed the research; C.C.L. and S.E.C. conducted experiments; and C.C.L. and P.G.S. wrote the paper.
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Cellitti, S., Geierstanger, B. et al. Efficient expression of tyrosine-sulfated proteins in E. coli using an expanded genetic code. Nat Protoc 4, 1784–1789 (2009). https://doi.org/10.1038/nprot.2009.188
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.188
This article is cited by
-
Tyrosine O-sulfation proteoforms affect HIV-1 monoclonal antibody potency
Scientific Reports (2022)
-
Deciphering protein post-translational modifications using chemical biology tools
Nature Reviews Chemistry (2020)
-
Genetically encoding phosphotyrosine and its nonhydrolyzable analog in bacteria
Nature Chemical Biology (2017)
-
A genetically encoded sulfotyrosine for VHR function research
Protein & Cell (2013)
-
Site-specific labeling of proteins with NMR-active unnatural amino acids
Journal of Biomolecular NMR (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.