Abstract
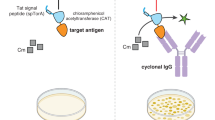
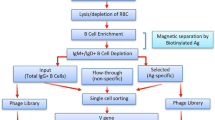
Here we describe a protocol for the selection of full-length IgG antibodies from repertoires displayed on Escherichia coli. In the method described here, full-length heavy and light chains are assembled in the periplasm into aglycosylated IgGs that are fully functional for antigen binding. Expression of an inner membrane-tethered Fc-binding protein is used to capture the IgG molecules and anchor them to the cell. Following outer-membrane permeabilization, fluorescently labeled ligand-binding library clones are selected by multiple rounds of fluorescence-activated cell sorting. Selection of a comprehensive set of IgG clones can typically be obtained within 3–4 weeks, a timescale that is comparable with most prevalent antibody display technologies. The isolated antibodies are well expressed in bacteria and exhibit affinities per binding site in the nanomolar range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Imai, K. & Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 6, 714–727 (2006).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Li, J. et al. Human antibodies for immunotherapy development generated via a human B cell hybridoma technology. Proc. Natl. Acad. Sci. USA 103, 3557–3562 (2006).
Fishwild, D.M. et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 14, 845–851 (1996).
Winter, G., Griffiths, A.D., Hawkins, R.E. & Hoogenboom, H.R. Making antibodies by phage display technology. Annu. Rev. Immunol. 12, 433–455 (1994).
Boder, E.T. & Wittrup, K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 15, 553–557 (1997).
Daugherty, P.S., Olsen, M.J., Iverson, B.L. & Georgiou, G. Development of an optimized expression system for the screening of antibody libraries displayed on the Escherichia coli surface. Protein Eng. 12, 613–621 (1999).
Lipovsek, D. & Pluckthun, A. In-vitro protein evolution by ribosome display and mRNA display. J. Immunol. Methods 290, 51–67 (2004).
Harvey, B.R. et al. Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries. Proc. Natl. Acad. Sci. USA 101, 9193–9198 (2004).
Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 23, 1105–1116 (2005).
Daugherty, P.S., Iverson, B.L. & Georgiou, G. Flow cytometric screening of cell-based libraries. J. Immunol. Methods 243, 211–227 (2000).
Hayhurst, A. & Georgiou, G. High-throughput antibody isolation. Curr. Opin. Chem. Biol. 5, 683–689 (2001).
Daugherty, P.S. Protein engineering with bacterial display. Curr. Opin. Struct. Biol. 17, 474–480 (2007).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Benhar, I. Design of synthetic antibody libraries. Expert Opin. Biol. Ther. 7, 763–779 (2007).
Chen, W. & Georgiou, G. Cell-surface display of heterologous proteins: from high-throughput screening to environmental applications. Biotechnol. Bioeng. 79, 496–503 (2002).
Samuelson, P., Gunneriusson, E., Nygren, P.A. & Stahl, S. Display of proteins on bacteria. J. Biotechnol. 96, 129–154 (2002).
Francisco, J.A., Earhart, C.F. & Georgiou, G. Transport and anchoring of beta-lactamase to the external surface of Escherichia coli . Proc. Natl. Acad. Sci. USA 89, 2713–2717 (1992).
Chen, G. et al. Isolation of high-affinity ligand-binding proteins by periplasmic expression with cytometric screening (PECS). Nat. Biotechnol. 19, 537–542 (2001).
Daugherty, P.S., Chen, G., Olsen, M.J., Iverson, B.L. & Georgiou, G. Antibody affinity maturation using bacterial surface display. Protein Eng. 11, 825–832 (1998).
Harvey, B.R. et al. Engineering of recombinant antibody fragments to methamphetamine by anchored periplasmic expression. J. Immunol. Methods 308, 43–52 (2006).
Jeong, K.J., Seo, M.J., Iverson, B.L. & Georgiou, G. APEx 2-hybrid, a quantitative protein–protein interaction assay for antibody discovery and engineering. Proc. Natl. Acad. Sci. USA 104, 8247–8252 (2007).
Worn, A. & Pluckthun, A. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305, 989–1010 (2001).
Mazor, Y., Blarcom, T.V., Mabry, R., Iverson, B.L. & Georgiou, G. Isolation of engineered, full-length antibodies from libraries expressed in Escherichia coli . Nat. Biotechnol. 25, 563–565 (2007).
Nilsson, B. et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1, 107–113 (1987).
Yu, F., Inouye, S. & Inouye, M. Lipoprotein-28, a cytoplasmic membrane lipoprotein from Escherichia coli. Cloning, DNA sequence, and expression of its gene. J. Biol. Chem. 261, 2284–2288 (1986).
Yamaguchi, K., Yu, F. & Inouye, M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli . Cell 53, 423–432 (1988).
Dane, K.Y., Chan, L.A., Rice, J.J. & Daugherty, P.S. Isolation of cell specific peptide ligands using fluorescent bacterial display libraries. J. Immunol. Methods 309, 120–129 (2006).
Akamatsu, Y., Pakabunto, K., Xu, Z., Zhang, Y. & Tsurushita, N. Whole IgG surface display on mammalian cells: Application to isolation of neutralizing chicken monoclonal anti-IL-12 antibodies. J. Immunol. Methods 327, 40–52 (2007).
Raju, T.S., Briggs, J.B., Borge, S.M. & Jones, A.J. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10, 477–486 (2000).
Radaev, S. & Sun, P.D. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J. Biol. Chem. 276, 16478–16483 (2001).
Jefferis, R. & Lund, J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol. Lett. 82, 57–65 (2002).
Simmons, L.C. et al. Expression of full-length immunoglobulins in Escherichia coli: rapid and efficient production of aglycosylated antibodies. J. Immunol. Methods 263, 133–147 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazor, Y., Van Blarcom, T., Iverson, B. et al. E-clonal antibodies: selection of full-length IgG antibodies using bacterial periplasmic display. Nat Protoc 3, 1766–1777 (2008). https://doi.org/10.1038/nprot.2008.176
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.176
This article is cited by
-
Isolation of full-length IgG antibodies from combinatorial libraries expressed in the cytoplasm of Escherichia coli
Nature Communications (2023)
-
Enzymatic Synthesis of Nucleoside Triphosphates and Deoxynucleoside Triphosphates by Surface-Displayed Kinases
Applied Biochemistry and Biotechnology (2020)
-
Antibody affinity maturation through combining display of two-chain paired antibody and precision flow cytometric sorting
Applied Microbiology and Biotechnology (2016)
-
Bacterial cytoplasm as an effective cell compartment for producing functional VHH-based affinity reagents and Camelidae IgG-like recombinant antibodies
Microbial Cell Factories (2014)
-
Creation of recombinant antigen-binding molecules derived from hybridomas secreting specific antibodies
Nature Protocols (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.