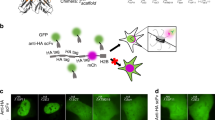
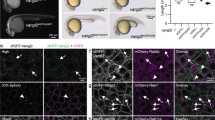
Abstract
Photoconvertible fluorescent proteins, such as Kaede, can be switched irreversibly from their native color to a new one. This property can be exploited to visualize de novo mRNA translation, because newly synthesized proteins can be distinguished from preexisting ones by their color. In this protocol, Kaede cDNA linked to the 3′ untranslated region (UTR) of β-actin is delivered into cells fated to become the retina by injection into Xenopus blastomeres. Brief exposure (6–10 s) to UV light (350–410 nm) of Kaede-positive retinal axons/growth cones efficiently converts Kaede from its native green fluorescence to red. The reappearance of the green signal reports the synthesis of new Kaede protein. This approach can be used to investigate the spatiotemporal control of translation of specific mRNAs in response to external stimuli and to test the efficiency of full-length versus mutant UTRs. The 3-d protocol can be adapted for broad use with other photoactivatable fluorescent proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Piper, M. & Holt, C. RNA translation in axons. Annu. Rev. Cell. Dev. Biol. 20, 505–523 (2004).
Steward, O. & Schuman, E.M. Compartmentalized synthesis and degradation of proteins in neurons. Neuron 40, 347–359 (2003).
Wilkie, G.S., Dickson, K.S. & Gray, N.K. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem. Sci. 28, 182–188 (2003).
Aakalu, G., Smith, W.B., Nguyen, N., Jiang, C. & Schuman, E.M. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30, 489–502 (2001).
Aronov, S., Aranda, G., Behar, L. & Ginzburg, I. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J. Cell Sci. 115, 3817–3827 (2002).
Brittis, P.A., Lu, Q. & Flanagan, J.G. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223–235 (2002).
Leung, K.M. et al. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 9, 1247–1256 (2006).
Wu, K.Y. et al. Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 (2005).
Kislauskis, E.H., Li, Z., Singer, R.H. & Taneja, K.L. Isoform-specific 3′-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J. Cell Biol. 123, 165–172 (1993).
Kislauskis, E.H., Zhu, X. & Singer, R.H. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J. Cell Biol. 127, 441–451 (1994).
Bassell, G.J. et al. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 18, 251–265 (1998).
Huttelmaier, S. et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438, 512–515 (2005).
Job, C. & Eberwine, J. Identification of sites for exponential translation in living dendrites. Proc. Natl. Acad. Sci. USA 98, 13037–13042 (2001).
Terskikh, A. et al. 'Fluorescent timer': protein that changes color with time. Science 290, 1585–1588 (2000).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S. & Verkhusha, V.V. Innovation: photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6, 885–891 (2005).
Raab-Graham, K.F., Haddick, P.C., Jan, Y.N. & Jan, L.Y. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science 314, 144–148 (2006).
Hatta, K., Tsujii, H. & Omura, T. Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 1, 960–967 (2006).
Bernas, T., Zarebski, M., Dobrucki, J.W. & Cook, P.R. Minimizing photobleaching during confocal microscopy of fluorescent probes bound to chromatin: role of anoxia and photon flux. J. Microsc. 215, 281–296 (2004).
Song, L., Varma, C.A., Verhoeven, J.W. & Tanke, H.J. Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopy. Biophys. J. 70, 2959–2968 (1996).
Song, L., van Gijlswijk, R.P., Young, I.T. & Tanke, H.J. Influence of fluorochrome labeling density on the photobleaching kinetics of fluorescein in microscopy. Cytometry 27, 213–223 (1997).
Dixit, R. & Cyr, R. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J. 36, 280–290 (2003).
Mizuno, H. et al. Photo-induced peptide cleavage in the green-to-red conversion of a fluorescent protein. Mol. Cell 12, 1051–1058 (2003).
Dittrich, P.S., Schafer, S.P. & Schwille, P. Characterization of the photoconversion on reaction of the fluorescent protein Kaede on the single-molecule level. Biophys. J. 89, 3446–3455 (2005).
Sato, T., Takahoko, M. & Okamoto, H. HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis 44, 136–142 (2006).
Stark, D.A. & Kulesa, P.M. An in vivo comparison of photoactivatable fluorescent proteins in an avian embryo model. Dev. Dyn. 236, 1583–1594 (2007).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Wachter, R.M. Chromogenic cross-link formation in green fluorescent protein. Acc. Chem. Res. 40, 120–127 (2007).
Nienhaus, G.U. et al. Photoconvertible fluorescent protein EosFP: biophysical properties and cell biology applications. Photochem Photobiol 82, 351–358 (2006).
Gurskaya, N.G. et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465 (2006).
Sahly, I., Erez, H., Khoutorsky, A., Shapira, E. & Spira, M.E. Effective expression of the green fluorescent fusion proteins in cultured Aplysia neurons. J. Neurosci. Methods 126, 111–117 (2003).
Vize, P.D., Melton, D.A., Hemmati-Brivanlou, A. & Harland, R.M. Assays for gene function in developing Xenopus embryos. Methods Cell Biol. 36, 367–387 (1991).
Etkin, L.D. & Pearman, B. Distribution, expression and germ line transmission of exogenous DNA sequences following microinjection into Xenopus laevis eggs. Development 99, 15–23 (1987).
Harland, R. & Misher, L. Stability of RNA in developing Xenopus embryos and identification of a destabilizing sequence in TFIIIA messenger RNA. Development 102, 837–852 (1988).
Holt, C.E., Garlick, N. & Cornel, E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron 4, 203 (1990).
Ohnuma, S., Mann, F., Boy, S., Perron, M. & Harris, W.A. Lipofection strategy for the study of Xenopus retinal development. Methods 28, 411–419 (2002).
Nieuwkoop, P.D. & Faber, J. The Normal Table of Xenopus laevis (Daudin) (Garland Publishing Inc., New York, 1994).
Sive, H.L., Grainger, R.M. & Harland, R. Early Development of Xenopus Laevis: a Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1998).
Shirasaki, R., Mirzayan, C., Tessier-Lavigne, M. & Murakami, F. Guidance of circumferentially growing axons by netrin-dependent and -independent floor plate chemotropism in the vertebrate brain. Neuron 17, 1079–1088 (1996).
Huang, S. & Moody, S.A. The retinal fate of Xenopus cleavage stage progenitors is dependent upon blastomere position and competence: studies of normal and regulated clones. J. Neurosci. 13, 3193–3210 (1993).
Acknowledgements
We thank M. Spira for suggesting the use of Kaede. We also thank M. Agathocleous, J. Falk, L. Leung, A. Lin and F. van Horck for critical reading of the manuscript. This work was supported by a Croucher Scholarship (K.-M.L.) and a Wellcome Trust Programme Grant (C.E.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, KM., Holt, C. Live visualization of protein synthesis in axonal growth cones by microinjection of photoconvertible Kaede into Xenopus embryos. Nat Protoc 3, 1318–1327 (2008). https://doi.org/10.1038/nprot.2008.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.113
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.