Abstract
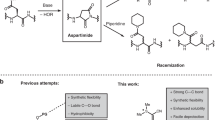
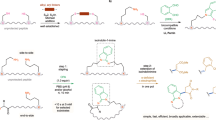
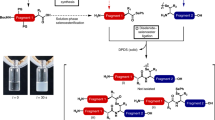
This protocol describes the methodology for the synthesis of dehydroalanine (Dha)-containing peptides and illustrates their use in convergent ligation strategies for the preparation of peptide conjugates. A nonproteinogenic amino acid, Fmoc-Se-phenylselenocysteine (SecPh), can be prepared in high yield over four synthetic steps and be conveniently incorporated into peptides by standard solid-phase peptide synthesis techniques. Globally deprotected peptides containing phenylselenocysteine can be converted to dehydrated peptides following a chemoselective, mild oxidation with hydrogen peroxide or sodium periodate (i.e., the phenylselenocysteine side chain is converted to that of Dha). Dha residues are electrophilic handles for the preparation of glycopeptides, lipopeptides or other peptide conjugates; one such transformation will be outlined here. The preparation of Dha-containing peptides, including the synthesis of SecPh, peptide elongation and oxidative treatment of phenylselenocysteine-containing peptides can be completed by one person in approximately 3–5 weeks. However, once SecPh is in hand, the time required for the preparation of peptides is significantly shorter and comparable to that for any peptide synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chatterjee, C., Paul, M., Xie, L. & van der Donk, W.A. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684 (2005).
Lau, R.C.M. & Rinehart, K.L. Berninamycin-B, Berninamycin-C, and Berninamycin-D, minor metabolites from Streptomyces bernensis. J. Antibiot. 47, 1466–1472 (1994).
Anderson, B., Hodgkin, D.C. & Viswamitra, M.A. The structure of thiostrepton. Nature 225, 233–235 (1970).
Galonić, D.P., van der Donk, W.A. & Gin, D.Y. Oligosaccharide-peptide ligation of glycosyl thiolates with dehydropeptides: synthesis of S-linked mucin-related glycopeptide conjugates. Chem. Eur. J. 9, 5997–6006 (2003).
Zhu, Y. & van der Donk, W.A. Convergent synthesis of peptide conjugates using dehydroalanines for chemoselective ligations. Org. Lett. 3, 1189–1192 (2001).
Gieselman, M.D., Zhu, Y., Zhou, H., Galonic, D. & van der Donk, W.A. Selenocysteine derivatives for chemoselective ligations. Chembiochem 3, 709–716 (2002).
Lemieux, G.A. & Bertozzi, C.R. Chemoselective ligation reactions with proteins, oligosaccharides and cells. TIBTECH 16, 506–513 (1998).
Durek, T. & Becker, C.F. Protein semi-synthesis: new proteins for functional and structural studies. Biomol. Eng. 22, 153–172 (2005).
Dawson, P.E., Muir, T.W., Clark-Lewis, I. & Kent, S.B.H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Muir, T.W., Sondhi, D. & Cole, P.A. Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. USA 95, 6705–6710 (1998).
Dawson, P.E. & Kent, S.B.H. Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 69, 923–960 (2000).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Saxon, E. & Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Nilsson, B.L., Kiessling, L.L. & Raines, R.T. Staudinger ligation: a peptide from a thioester and azide. Org. Lett. 2, 1939–1941 (2000).
Galonić, D.P., van der Donk, W.A. & Gin, D.Y. Site-selective conjugation of thiols with aziridine-2-carboxylic acid-containing peptides. J. Am. Chem. Soc. 126, 12712–12713 (2004).
Gamblin, D.P. et al. Glyco-SeS: selenenylsulfide-mediated protein glycoconjugation––a new strategy in post-translational modification. Angew. Chem. Int. Ed. 43, 828–833 (2004).
Davis, B.G. Biochemistry. Mimicking posttranslational modifications of proteins. Science 303, 480–482 (2004).
Schmidt, U., Lieberknecht, A. & Wild, J. Didehydroamino acids (DDAA) and didehydropeptides (DDP). Synthesis 159–172 (1988).
Ranganathan, D., Shah, K. & Vaish, N. An exceptionally mild and efficient route to dehydroalanine peptides. J. Chem. Soc. Chem. Commun. 1145–1147 (1992).
Blettner, C. & Bradley, M. Asparagine as a masked dehydroalanineresidue in solid-phase peptide-synthesis. Tetrahedron Lett. 35, 467–470 (1994).
Burrage, S. et al. Biomimetic synthesis of lantibiotics. Chem. Eur. J. 6, 1455–1466 (2000).
Okeley, N.M., Zhu, Y. & van der Donk, W.A. Facile chemoselective synthesis of dehydroalanine-containing peptides. Org. Lett. 2, 3603–3606 (2000).
Hashimoto, K., Sakai, M., Okuno, T. & Shirahama, H. β-Phenylselenoalanine as a dehydroalanine precursor in the efficient synthesis of alternariolide (AM-toxinI). Chem. Commun. 1139–1140 (1996).
Zhou, H. & van der Donk, W.A. Biomimetic stereoselective formation of methyllanthionine. Org. Lett. 4, 1335–1338 (2002).
Zhu, Y., Gieselman, M., Zhou, H., Averin, O. & van der Donk, W.A. Biomimetic studies on the mechanism of stereoselective lanthionine formation. Org. Biomol. Chem. 1, 3304–3315 (2003).
Ley, S.V., Priour, A. & Heusser, C. Total synthesis of the cyclic heptapeptide Argyrin B: a new potent inhibitor of T-cell independent antibody formation. Org. Lett. 4, 711–714 (2002).
Snider, B.B. & Zeng, H. Total syntheses of (−)−fumiquinazolines C, E, and H. Org. Lett. 4, 1087–1090 (2002).
Nicolaou, K.C., Zak, M., Safina, B.S., Lee, S.H. & Estrada, A.A. Total synthesis of thiostrepton, part 2: construction of the quinaldic acid macrocycle and final stages of the synthesis. Angew. Chem. Int. Ed. Engl. 43, 5092–5097 (2004).
Seebeck, F.P. & Szostak, J.W. Ribosomal synthesis of dehydroalanine-containing peptides. J. Am. Chem. Soc. 128, 7150–7151 (2006).
Kluskens, L.D. et al. Post-translational modification of therapeutic peptides by NisB, the dehydratase of the lantibiotic nisin. Biochemistry 44, 12827–12834 (2005).
Chatterjee, C., Patton, G.C., Cooper, L., Paul, M. & van der Donk, W.A. Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13, 1109–1117 (2006).
Reich, H.J., Jasperse, C.P. & Renga, J.M. Organoselenium chemistry. Alkylation of acid, ester, amide, and ketone enolates with bromomethyl benzylselenide and sulfide: preparation of selenocysteine derivatives. J. Org. Chem. 51, 2981 (1986).
Stocking, E.M., Schwarz, J.N., Senn, H., Salzmann, M. & Silks,, L.A. Synthesis of L-selenocystine, L-[se-77]selenocystine and L-tellurocystine. J. Chem. Soc. Perkin Trans. 1 2443–2447 (1997).
Gieselman, M.D., Xie, L. & van der Donk, W.A. Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org. Lett. 3, 1331–1334 (2001).
Bhat, R.G., Porhiel, E., Saravanan, V. & Chandrasekaran, S. Utility of tetrathiomolybdate and tetraselenotungstate: efficient synthesis of cystine, selenocystine, and their higher homologues. Tetrahedron Lett. 44, 5251–5253 (2003).
Siebum, A.H.G., Woo, W.S., Raap, J. & Lugtenburg, J. Access to any site-directed isotopomer of methionine, selenomethionine, cysteine, and selenocysteine––use of simple, efficient modular synthetic reaction schemes for isotope incorporation. Eur. J. Org. Chem. 2905–2913 (2004).
Braga, A.L., Vargas, F., Sehnem, J.A. & Braga, R.C. Efficient synthesis of chiral beta-seleno amides via ring-opening reaction of 2-oxazolines and their application in the palladium-catalyzed asymmetric allylic alkylation. J. Org. Chem. 70, 9021–9024 (2005).
Braga, A.L. et al. Chiral seleno-amines from indium selenolates. A straightforward synthesis of selenocysteine derivatives. J. Org. Chem. 71, 4305–4307 (2006).
Still, C.W., Kahn, M. & Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 43, 2923–2925 (1978).
Han, Y., Albericio, F. & Barany, G. Occurrence and minimization of cysteine racemization during stepwise solid-phase peptide synthesis. J. Org. Chem. 62, 4307–4312 (1997).
Jeffrey, P.D. & McCombie, S.W. Homogeneous, palladium(0)-catalyzed exchange deprotection of allyl esters, carbonates, and carbamates. J. Org. Chem. 47, 587–590 (1982).
Besse, D. & Moroder, L. Synthesis of selenocysteine peptides and their oxidation to diselenide bridged compounds. J. Pept. Sci. 3, 442–453 (1997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Levengood, M., van der Donk, W. Dehydroalanine-containing peptides: preparation from phenylselenocysteine and utility in convergent ligation strategies. Nat Protoc 1, 3001–3010 (2006). https://doi.org/10.1038/nprot.2006.470
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.470
This article is cited by
-
Selenium chemistry for spatio-selective peptide and protein functionalization
Nature Reviews Chemistry (2024)
-
Studies on syntheses and self-assembly behaviour of homoseleno-peptides
Journal of Chemical Sciences (2023)
-
Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine
Nature Communications (2022)
-
Synthesis of selenopeptides: an alternative way of incorporating selenocystine
Amino Acids (2019)
-
Post-translational mutagenesis for installation of natural and unnatural amino acid side chains into recombinant proteins
Nature Protocols (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.