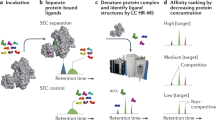
Abstract
This protocol describes a robust method for the covalent capture of small molecules with diverse reactive functional groups in microarray format, and outlines a procedure for probing small-molecule microarrays (SMMs) with proteins of interest. A vapor-catalyzed, isocyanate-mediated surface immobilization scheme is used to attach bioactive small molecules, natural products and small molecules derived from diversity-oriented synthesis pathways. Additionally, an optimized methodology for screening SMMs with purified proteins and cellular lysates is described. Finally, a suggested model for data analysis that is compatible with commercially available software is provided. These procedures enable a platform capability for discovering novel interactions with potential applications to immunoglobulin profiling, comparative analysis of cellular states and ligand discovery. With the appropriate materials and experimental setup, the printing of SMMs can be completed in 14 hours over 3 days. Screening and data analysis requires 2 days. A detailed timeline is provided.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lu, F. et al. Proteomic libraries: design, synthesis, and evaluation of p53-MDM2 interaction inhibitors. J. Comb. Chem. 8, 315–325 (2006).
Katragadda, M., Magotti, P., Sfyroera, G. & Lambris, J.D. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 49, 4616–4622 (2006).
Lo, M.C. et al. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 332, 153–159 (2004).
Navratilova, I., Dioszegi, M. & Myszka, D.G. Analyzing ligand and small molecule binding activity of solubilized GPCRs using biosensor technology. Anal. Biochem. 355, 132–139 (2006).
Clemons, P.A. et al. Synthesis of calcineurin-resistant derivatives of FK506 and selection of compensatory receptors. Chem. Biol. 9, 49–61 (2002).
Peng, L. et al. Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat. Chem. Biol. 2, 381–389 (2006).
Moll, D. et al. Biomolecular interaction analysis in functional proteomics. J. Neural Transm. 113, 1015–1032 (2006).
MacBeath, G., Koehler, A.N. & Schreiber, S.L. Printing small molecules as microarrays and detecting protein–small molecule interactions en masse. J. Am. Chem. Soc. 121, 7967–7968 (1999).
Hergenrother, P.J., Depew, K.M. & Schreiber, S.L. Small molecule microarrays: covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 122, 7849–7850 (2000).
Barnes-Seeman, D., Park, S.B., Koehler, A.N. & Schreiber, S.L. Expanding the functional group compatibility of small-molecule microarrays: discovery or novel calmodulin ligands. Angew. Chem. Int. Ed. Engl. 42, 2376–2379 (2003).
Houseman, B.T. & Mrksich, M. Carbohydrate arrays for the evaluation of protein binding and enzymatic modification. Chem. Biol. 9, 443–454 (2002).
Kanoh, N. et al. Immobilization of natural products on glass slides using a photoaffinity reaction and the detection of protein–small molecule interactions. Angew. Chem. Int. Ed. Engl. 42, 5584–5587 (2003).
Kohn, M. et al. Staudinger ligation: a new immobilization strategy for the preparation of small-molecule arrays. Angew. Chem. Int. Ed. Engl. 42, 5830–5834 (2003).
Uttamchandani, M. et al. Microarrays of tagged combinatorial triazine libraries in the discovery of small molecule ligands of human IgG. J. Comb. Chem. 6, 862–868 (2004).
Lee, M.R. & Shin, I. Fabrication of chemical microarrays by efficient immobilization of hydrazide-linked substances on epoxide-coated glass surfaces. Angew. Chem. Int. Ed. Engl. 44, 2881–2884 (2005).
Bradner, J.E. et al. A robust small-molecule microarray platform for screening cell lysates. Chem. Biol. 13, 493–504 (2006).
National Research Council. Prudent Practices for Handling Hazardous Chemicals (The National Academies Press, Washington DC, 1995).
Keenan, T. et al. Synthesis and activity of bivalent FKBP12 ligands for the regulated dimerization of proteins. Bioorg. Med. Chem. 6, 1309–1335 (1998).
Acknowledgements
The project has been funded in whole or in part with Federal funds from the National Cancer Institute's Initiative for Chemical Genetics, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Service, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. We thank R. Mazitschek for donation of AP1497 derivatives. J.E.B. is supported by the Multiple Myeloma Research Foundation and the Clinical Investigator Training Program: Harvard–MIT Health Sciences and Technology–Beth Israel Deaconess Medical Center, in collaboration with Pfizer and Merck & Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Parafilm Screening Methods. (AVI 3475 kb)
Supplementary Video 2
Small Molecule Microarrays (MOV 5969 kb)
Rights and permissions
About this article
Cite this article
Bradner, J., McPherson, O. & Koehler, A. A method for the covalent capture and screening of diverse small molecules in a microarray format. Nat Protoc 1, 2344–2352 (2006). https://doi.org/10.1038/nprot.2006.282
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.282
This article is cited by
-
Advances in targeting ‘undruggable’ transcription factors with small molecules
Nature Reviews Drug Discovery (2021)
-
Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure
Nature Communications (2019)
-
Targeting transcription is no longer a quixotic quest
Nature Chemical Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.