Abstract
GABAergic signaling is involved in modulating the reinforcing properties of alcohol, and GABAB receptors have been proposed as a potential target for clinical treatment of alcoholism. The orthosteric GABAB receptor agonist baclofen has been shown to suppress operant self-administration of alcohol in animals and alcohol use in alcohol-dependent patients, but its utility is limited by a narrow therapeutic index. We tested the effects of ADX71441, a novel GABAB receptor positive allosteric modulator, on alcohol-related behaviors in rats. We first assessed the effects of ADX71441 (1, 3, 10 and 30 mg/kg, I.P.) on both non-dependent and dependent male Wistar rats trained to self-administer 20% alcohol. We then determined the effects of ADX71441 on stress-induced as well as cue-induced relapse-like behavior. Finally, we sought to identify the brain regions through which ADX71441 may act to prevent relapse-like behavior by mapping the neuronal activation induced by stress-induced reinstatement of alcohol-seeking using c-Fos immunohistochemistry. ADX71441 dose-dependently decreased alcohol self-administration of both dependent and non-dependent animals, but its potency was higher in alcohol-dependent rats. Furthermore, both cue- and stress-induced alcohol seeking were blocked by the GABAB receptor positive allosteric modulator. Finally, pretreatment with 3 mg/kg of ADX71441 before stress-induced reinstatement significantly decreased c-Fos expression in a network of brain regions implicated in stress-induced relapse, comprising the nucleus accumbens shell, the dorsal raphe nucleus and the medial prefrontal cortex. Our findings support a causal role of GABAB receptors in alcohol reinforcement and relapse to alcohol seeking. These effects are observed in the absence of significant sedative side effects. Jointly, these observations indicate that GABAB receptor positive allosteric modulators merit being tested clinically for the treatment of alcoholism. Our data also point to a potential biomarker of target engagement for early clinical studies.
Similar content being viewed by others
Introduction
Gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the brain (Bormann, 2000; Sivilotti and Nistri, 1991) is key for the development and maintenance of drug addiction. The synaptic actions of GABA are mediated by two major classes of receptors: the ionotropic receptors GABAA and GABAC which selectively increase chloride conductance, and the metabotropic receptors (GABAB), which increase potassium conductance (Bormann, 2000; Dutar and Nicoll, 1988). GABAB receptors have been proposed as a potential target for clinical treatment of addiction (Addolorato et al, 2009; Brebner et al, 2002; Cousins et al, 2002; Heilig and Egli, 2006). Numerous reports have indicated that GABAB receptor agonists can affect addiction-related behaviors in preclinical models of alcoholism and other addictive disorders. For example, the orthosteric GABAB receptor agonist baclofen, in clinical use to treat spasticity, has been shown to attenuate the reinforcing effects of cocaine and the motivation to self-administer this drug in rodents (Roberts and Andrews, 1997; Roberts et al, 1996). Baclofen also dose-dependently reduced nicotine-, morphine- and cocaine-induced dopamine release in the nucleus accumbens shell (NAcSh; Fadda et al, 2003).
The most extensive data for an involvement of GABAB receptors are available for alcohol-related behaviors. Baclofen was first shown to be of potential value for the treatment of alcoholism in mice. These experiments showed that an acute injection of a dose of 5 mg/kg of baclofen suppressed ethanol-induced locomotor stimulation (Cott et al, 1976). Furthermore, baclofen but not muscimol (a GABAA receptor agonist) decreased voluntary ethanol consumption in a two-bottle choice paradigm (Daoust et al, 1987), suggesting a selective role of metabotropic GABAB receptors in modulation of alcohol intake. Following these seminal studies, numerous reports provided further support for the notion that baclofen may be a candidate medication for alcoholism treatment. For instance, baclofen was shown to reduce operant alcohol self-administration and motivation for alcohol in non-dependent rats (Anstrom et al, 2003; Janak and Michael Gill, 2003), dependent rats (Walker and Koob, 2007) as well as selectively bred strains of alcohol-preferring rats (Liang et al, 2006; Maccioni et al, 2005). In addition, it prevented the acquisition of alcohol drinking (Colombo et al, 2002) and suppressed extinction responding for alcohol in Sardinian alcohol-preferring rats (Colombo et al, 2003).
Baclofen has also shown promise in clinical studies. An initial study in 5 patients suggested that administration of low baclofen doses (30 mg/day) rapidly suppressed severe symptoms of alcohol withdrawal syndrome (Addolorato et al, 2002b), and patients were able to maintain abstinence during a 30-day follow-up period. A randomized controlled trial (RCT) that followed investigated the efficacy of baclofen to suppress drinking over a month in 39 alcohol-dependent subjects (Addolorato et al, 2002a), and found that baclofen reduced alcohol intake and craving, increased the number of cumulative abstinence days as well as the proportion of patients abstinent from alcohol. Finally, an ambitious RCT in severely alcohol-dependent patients with liver disease found that baclofen more than doubled the proportion of participants who achieved abstinence and maintained it for the 12 week duration of the study. A twofold increase in cumulative abstinence duration was also found (Addolorato et al, 2007). In contrast, an RCT conducted in the U.S. (Garbutt et al, 2010) was negative. A possible reason for this apparent discrepancy is that the Italian study recruited patients with more severe alcohol use disorder (14 mean daily drinks vs 7 in the U.S. study), which suggests that baclofen may be more efficacious in heavily dependent patients (Leggio et al, 2010).
Despite promising results in both clinical and preclinical models, the use of baclofen remains controversial, and the drug is still not approved by the Food and Drug Administration (FDA) or European Medicines Agency (EMA) as a treatment for alcoholism or other substance use disorders. A key reason for this is that the use of baclofen is limited by side effects. For example, while baclofen successfully reduced alcohol self-administration in C57BL/6J, doses that suppressed the reinforcing effects of alcohol also suppressed locomotion and potentiated the sedative effects of ethanol, even at alcohol doses that were not sedative per se (Besheer et al, 2004). In a clinical trial in cocaine abusing male patients, baclofen administration was associated with headache, nausea, sedation and dizziness (Ling et al, 1998).
GABAB receptor-positive allosteric modulators (PAM:s) may help to overcome these limitations (Pin and Prezeau, 2007). PAM:s exert their effects on GABAB receptors through allosteric binding to a site that is topographically distinct from that bound by the endogenous ligand (orthosteric site; Conn et al, 2009; May and Christopoulos, 2003). Upon binding, PAM:s lack intrinsic activity, and act by potentiating the signaling that is triggered when the natural ligand (GABA) activates the receptor. This mechanism offers several advantages over orthosteric agonists, and has been proposed to enable PAM:s to produce less adverse effects and lead to lower tolerance than direct agonists (May and Christopoulos, 2003; Perdona et al, 2011; Urwyler, 2011).
Here, we therefore assessed the effects of ADX71441, a novel GABAB PAM that has been recently shown to reduce ethanol drinking in mice (Hwa et al, 2014) and has entered Phase 1 clinical testing, on alcohol-related behaviors in rats. We first trained Wistar rats to self-administer 20% alcohol, and tested the effects of ADX71441 on alcohol self-administration as well as motivation to self-administer alcohol. Using alcohol vapor exposure, a model of dependence to alcohol (Macey et al, 1996; Meinhardt and Sommer, 2015), we also compared the efficacy of ADX71441 to reduce alcohol reinforcing properties in non-dependent vs dependent animals. We then assessed the effects of ADX71441 on cue-induced as well as stress-induced alcohol seeking, a model of relapse (Epstein et al, 2006). Finally, we used c-Fos mapping to identify a neural signature of ADX71441 actions that could inform the development of a translational biomarker to be used in early clinical development (Heilig et al, 2016).
Materials and methods
Drugs
ADX71441 was obtained from Addex Therapeutics. The compound was suspended in a solution of 1% carboxymethyl cellulose (CMC) in water and was injected intraperitoneally at a volume of 1 or 3 ml/kg. Initial doses (1, 3, 10 mg/kg and 30 mg/kg) were chosen based on previous investigation (Kalinichev et al, 2014). Alcohol solutions were prepared volume/volume in tap water from 95% alcohol.
Subjects
A total of 101 adult male Wistar rats (Charles River, Frederick, MD, USA) weighing 200–225 g at the beginning of the experiments were pair-housed in a temperature- (21 °C) and humidity-controlled environment with a reverse 12 h light–dark cycle. Rats were given free access to chow and tap water for the duration of the experiment. All behavioral testing was conducted during the dark phase of the light–dark cycle. A detailed timeline of experimental training and testing can be found in Supplementary Figure 1. The studies were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Behavioral Testing
32 rats (group 1, see Supplementary Figure 1) were first trained to self-administer 20% (v/v) alcohol without sucrose/saccharin fading as described previously (Augier et al, 2017; Augier et al, 2014), first on a fixed ratio 1 (FR1) for 20 sessions, followed by 22 sessions on a FR2. They were then subjected to a battery of alcohol-associated behavioral tests: extinction, cue-induced reinstatement, stress-induced reinstatement and progressive ratio as described previously (Augier et al, 2016). Finally, rats were trained to self-administer 0.2% saccharin on a FR2. Detailed information is provided in Supplementary Methods section.
Dependence Induction
A separate group of rats (group 3, n=14) was exposed to alcohol vapor using chronic intermittent alcohol vapor exposure (Rimondini et al, 2002; Rogers et al, 1979). Briefly, rats were allowed to habituate to the chambers for 1 week and then exposed to alcohol vapor for 14 h a day (on at 7:30pm, off at 9:30 am) for 8 weeks, resulting in blood alcohol concentrations (BACs) between 150 and 250 mg/dl. Controls (n=16) were kept in identical chambers with normal air flow. Blood was collected weekly from the lateral tail vein and BACs were assessed using quantitative gas chromatography (Augier et al, 2017).
Brain Tissue Processing and Immunohistochemistry
Standard immunohistochemistry procedures were used for the c-Fos mapping study. Detailed information is provided in Supplementary Methods section.
Statistics
The effects of ADX71441 on alcohol self-administration, saccharin self-administration and locomotion were analyzed using a one-way repeated measures analysis of variance (one-way RM ANOVA). The effect of ADX71441 on progressive ratio responding was analyzed using a one-way ANOVA. The effects of ADX71441 on stress- and cue-induced reinstatement were analyzed using a two-way repeated measures analysis of variance (two-way RM ANOVA). Extinction data presented in Figure 3 were collapsed across groups to improve the readability of these figures. All groups were however compared and separately analyzed in the statistical analysis. All post hoc analyses were conducted when appropriate using a Tukey-HSD test.
Data for c-Fos-positive cells are reported as mean values±standard error as number per square millimeter. The data from each experiment were analyzed by t-test. Correlation analysis between active lever presses during the stress-induced reinstatement test and c-Fos expression in regions of interest were performed using the Pearson correlation coefficient value. For all statistical analysis, differences between control and experimental groups were considered significant if p<0.05.
Results
ADX71441 Potently Decreases Alcohol Self-Administration
Once operant responding had stabilized, the rats earned a total of 30.9±2.2 reinforcers and produced 77.2±5.9 presses on the active alcohol lever (Figure 1a and b respectively, white bars). One-way RM ANOVA analysis showed that ADX71441 dose-dependently decreased both alcohol reinforcers (F5,150=47.80, p<0.001; η2=0.61, Figure 1a) and active lever presses (F5,150=47.59, p<0.001; η2=0.61, Figure 1b). Post hoc analyses showed a significant effect of the 3, 10 and 30 mg/kg doses on active responses and reinforcers compared to vehicle (p<0.001 for all doses), but not an effect of the low dose of 1 mg/kg (p=0.25 for reinforcers and p=0.11 for active lever presses). The vehicle did not affect self-administration compared to baseline (p⩾0.99 for both reinforcers and lever presses).
ADX71441 strongly decreases 20% alcohol self-administration. (a) Mean reinforcers (±SEM) earned during a 30-min FR2 self-administration session of 20% EtOH following either vehicle or ADX71441 treatment (1, 3, 10 or 30 mg/kg; n=32; *p<0.001). (b) Mean active lever presses (±SEM) completed during a 30-min FR2 self-administration session of 20% EtOH following either vehicle or ADX71441 treatment (1, 3, 10 or 30 mg/kg; n=32; *p<0.001). (c) Mean distance traveled (±SEM) following either vehicle or ADX71441 treatment (1, 3, 10 or 30 mg/kg; n=18; *p<0.001). (d) Mean breakpoint (±SEM) reached during a progressive ratio session of 20% EtOH following either vehicle or ADX71441 treatment (3 or 10 mg/kg; n=10, 11 by groups; *p<0.001).
To examine whether this decrease of alcohol self-administration was the result of non-specific sedative or otherwise performance-impairing properties of the drug, the effect of ADX71441 on locomotor activity was assessed in a separate group of rats (Figure 1c). There was a significant effect of drug treatment (F4,68=12.86, p<0.001; η2=0.43). However, post hoc tests showed that only the 30 mg/kg dose was sedative (p<0.001); none of the other doses tested (1, 3 and 10 mg/kg) altered locomotor activity compared to the vehicle (p⩾0.97 for the three doses). In addition, there was no difference between baseline locomotion and the vehicle (p>0.99). Because of these data, the dose of 30 mg/kg was subsequently discontinued from the study.
The effect of ADX71441 on the motivation of the animals to consume alcohol was also tested using a progressive ratio reinforcement schedule (Hodos, 1961). The drug potently reduced alcohol breakpoints (F2,62=54.07, p<0.001; η2=0.64, Figure 1d) at both doses tested (3 and 10 mg/kg).
The Potency of ADX71441 to Suppress Alcohol Self-Administration is Enhanced in Rats with a History of Alcohol Dependence
A separate group of rats was exposed to intermittent alcohol vapor for 8 weeks (Figure 2a), resulting in mean BACs of 232.8±20.3 mg/dl during the vapor exposure (Supplementary Table 1). In agreement with prior reports (see (Meinhardt and Sommer, 2015) for review), once reintroduced to self-administration, vapor-exposed rats escalated their alcohol intake over 11 consecutive sessions compared to unexposed controls (Figure 2b and c). Post-dependent self-administration was stable over time (no main effect of session: F10,280=0.94, p=0.49; η2=0.03 for reinforcers; F10,280=0.92, p=0.51; η2=0.03 for active lever presses). There was a significant main effect of vapor exposure (F1,28=20.58, p<0.001; η2=0.42 for reinforcers; F1,28=15.17, p<0.001; η2=0.35 for active lever presses) as well as an interaction between vapor exposure and sessions (F10,280=1.83, p<0.05; η2=0.07 for reinforcers; F10,280=2.14, p<0.05; η2=0.07 for active lever presses). A post hoc comparison indicated that vapor-exposed rats significantly consumed more alcohol compared to unexposed controls during sessions 2, 3, 4, 5, 8 and 10 (p<0.05 for sessions 2, 8 and 10 and p<0.01 for sessions 3–5).
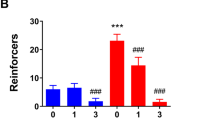
Rats with a history of dependence show an enhanced sensitivity to suppression of alcohol self-administration by ADX71441. (a) Schematic representation of the timeline of the experiment. (b) Mean reinforcers (±SEM) earned during eleven consecutive 30-min FR3 self-administration sessions of 20% EtOH following vapor exposure (n=16 and 14 by groups; *p<0.05). (c) Mean active lever presses (±SEM) completed during 11 consecutive 30-min FR3 self-administration sessions of 20% EtOH following vapor exposure (n=16 and 14 by groups; *p<0.05). (d) Mean reinforcers (±SEM) earned during a 30-min FR3 self-administration session of 20% EtOH following either vehicle or ADX71441 treatment (1 or 3 mg/kg; n=16 and 14 by groups; *p<0.05). (e) Mean active lever presses (±SEM) completed during a 30-min FR3 self-administration session of 20% EtOH following either vehicle or ADX71441 treatment (1 or 3 mg/kg; n=16 and 14 by groups; #p<0.05 compared to baseline, *p<0.05 compared to respective vehicle).
We then compared the effect of ADX71441 on 20% alcohol self-administration of both dependent and non-dependent rats (Figure 2d and e). There was a main effect of vapor exposure (F1,28=6.63, p<0.05; η2=0.19 for reinforcers; F1,28=6.08, p<0.05; η2=0.20 for active lever presses), a main effect of ADX71441 treatment (F3,84=34.51, p<0.001; η2=0.55 for reinforcers; F3,84=29.88, p<0.001; η2=0.54 for active lever presses), and a significant interaction between vapor exposure and ADX71441 treatment (F3,84=2.99, p<0.05; η2=0.10 for reinforcers; F3,84=3.13, p<0.05; η2=0.11 for active lever presses). Post hoc analyses showed that the lowest dose of 1 mg/kg decreased alcohol self-administration in post-dependent animals (p<0.05 for both reinforcers and active lever presses), but not in non-dependent controls (p=0.65 for reinforcers and p=0.59 for active lever presses), showing that post-dependent animals were more sensitive to the drug. The 3 mg/kg dose significantly reduced alcohol self-administration in both groups (p<0.001 for vapor-exposed animals and p<0.01 for controls for reinforcers; p<0.001 for both groups for active lever presses).
Of note, differential sensitivity to drug effects in dependent and non-dependent rats might be related to their differential response rates. This is, however, unlikely to be the case here; with prolonged training, non-dependent rats 1 in Experiment 1 and the dependent rats in Experiment 2 reached similar response rates, yet reduction in responding for alcohol was not observed in the non-dependent animals until the dose reached 3 mg/kg.
ADX71441 Potently Blocks Both Cue- and Stress-Induced Alcohol-Seeking
Following extinction, rats successfully extinguished their responding for alcohol (Figure 3a). There was no difference in extinction responding rates between the three groups (all p values ⩾0.95). There was a significant main effect of test condition, demonstrating a robust reinstatement of responding on the previously alcohol-associated level (F1,27=4.76, p<0.05; η2=0.15). This was confirmed on post hoc analysis, which showed that reintroduction of the alcohol-associated cues led to a robust reinstatement of alcohol-seeking in the vehicle group (p<0.001). There was a significant main effect of ADX71441 treatment (F2,27=18.21, p<0.001; η2=0.57), and a significant interaction between test condition and treatment (F2,27=12.65, p<0.001; η2=0.48). Specifically, post hoc analysis showed that treatment with ADX71441 blocked cue-induced reinstatement (Figure 3a, p<0.001 for both 3 and 10 mg/kg).
ADX71441 blocks both cue-induced and stress-induced alcohol-seeking (a) mean number of non-reinforced lever presses (±SEM) during the 30-min test for cue-induced reinstatement following either saline or ADX71441 treatment (3 or 10 mg/kg; n=10 by groups; *p<0.001 compared to vehicle, #p<0.001 compared to extinction). (b) Mean number of non-reinforced lever presses (±SEM) during the 30-min test for stress-induced reinstatement following either saline or ADX71441 treatment (3 or 10 mg/kg; n=10, 11 by groups; *p<0.01 compared to vehicle, #p<0.01 compared to extinction).
Following cue-induced reinstatement testing, responding for alcohol was re-extinguished through additional extinction sessions. Responses on the active lever decreased to a low level (reaching an average of 12.9±1.1 through the 3 last sessions of extinction, Figure 3b). Exposure to the footshock stressor led to a robust reinstatement of alcohol-seeking (main effect of the reinstatement test, F1,27=7.29, p<0.05; η2=0.21) in the vehicle-treated animals (p<0.01). There was a main effect of ADX71441 treatment (F2,27=6.58, p<0.01; η2=0.33) and a significant interaction between test condition and treatment (F2,27=8.31, p<0.01; η2=0.38). In detail, both doses of 3 and 10 mg/kg potently blocked stress-induced alcohol seeking (p<0.01 and p<0.001 respectively).
ADX71441 Also Suppresses Operant Responding for the Non-Caloric Reinforcer Saccharin
Rats previously trained on FR2 alcohol self-administration quickly acquired FR2 0.2% saccharin self-administration. They earned 130.1±9.8 reinforcers by session (Supplementary Figure 2a) and produced 341.8±31.8 responses on the active lever (Supplementary Figure 2b). ADX71441 dose-dependently decreased both saccharin reinforcers (F4,116=47.30, p<0.001; η2=0.62, Supplementary Figure 2a) and active lever presses (F4,116=44.90, p<0.001; η2=0.61, Supplementary Figure 2b). In addition, post hoc analyses showed a significant effect of the 3 and 10 mg/kg doses on both responses and saccharin reinforcers earned compared to vehicle (p<0.001 for both doses). There was no difference between baseline and vehicle (p⩾0.99 for both reinforcers and lever presses).
ADX71441 Attenuates Stress-Induced Neuronal Activity in an Interconnected Network of Brain Structures
We used c-Fos mapping to search for the neural substrates through which ADX71441 may act to prevent stress-induced relapse to alcohol seeking. A separate group of rats was trained to self-administer 20% alcohol, allowed to reach a stable baseline, extinction trained and then tested for stress-induced reinstatement. In a replication of the initial experiment, a dose of 3 mg/kg of ADX71441 potently blocked stress-induced reinstatement of alcohol seeking (Supplementary Figure 3). Ninety minutes after the start of the reinstatement session, rats in this group were perfused and brains were collected for analysis of c-Fos immunoreactivity, performed in a set of brain regions previously shown to be associated with stress-induced relapse to alcohol seeking (Funk et al, 2006; Schank et al, 2015): the medial prefrontal cortex (mPFC), NAcSh, bed nucleus of stria terminalis (BNST), central amygdala (CeA), basolateral amygdala (BLA) and dorsal raphe nucleus (DRN).
A dose of ADX71441 that in prior experiments suppressed stress-induced reinstatement without producing sedation, 3 mg/kg, significantly decreased c-Fos expression in several brain structures when these were examined individually: the NAcSh (Figure 4a, t (19)=2.52, p=0.02), CeA (Figure 4b, t (17)=2.76, p=0.01), and DRN (Figure 4c, t (17)=2.20, p=0.04). There was also a trend for an effect of ADX71441 on c-Fos levels in the mPFC (Figure 4d, t (19)=1.89, p=0.08). In contrast, there was no effect of treatment in the BNST (Figure 4e, t (19)=1.04, p=0.29) or BLA (Figure 4f, t (19)=1.06, p=0.41).
ADX71441 attenuates stress-induced neuronal activity in the nucleus accumbens shell, central amygdala and dorsal raphe nucleus. Mean c-Fos-positive cells (±SEM) following either vehicle or ADX71441 treatment (3 mg/kg) in the (a) nucleus accumbens shell (NAcSh), (b) central amygdala (CeA), (c) dorsal raphe nucleus (DRN), (d) medial prefrontal cortex (mPFC), (e) bed nucleus of stria terminalis (BNST) and (f) basolateral amygdala (BLA; n=9–11 by groups; *p<0.05). Representative images are shown at × 40 magnification.
We then examined whether ADX71441 may block stress-induced reinstatement by inhibiting a network of interconnected stress-responsive brain structures; identifying such a network has the potential to inform the development of translational biomarkers for early clinical studies. To this end, we carried out a principal component extraction, followed by a factor analysis. The full correlation table of c-Fos expression in the brain structures examined is provided in Supplementary Table 2. Two factors with eigenvalues >1 were obtained. Following normalized varimax rotation, these jointly accounted for 76.4% of total variance in c-Fos data. c-Fos activity in the NAcSh (factor loading: 0.92), mPFC (0.90) and DRN (0.84) loaded strongly on the first of these factor (‘Network 1’), and accounted for 42.5% of total variance. In contrast, activity in BLA (0.89), BNST (0.73) and CeA (0.71) loaded on a second factor (‘Network 2’), and accounted for 34% of total variance.
Activation of Network 1 showed a strong correlation with reinstatement responding (R2=0.71, p=0.00002; Figure 5a), suggesting that it may causally contribute to stress-induced relapse. This correlation was driven by correlation of each of these 3 structures with reinstatement responding (Supplementary Figure 4). Accordingly, ADX71441 treatment significantly reduced Network 1 activation, with a large effect size (main effect of treatment on factor scores: F1,15=6.62, p=0.02; η2=0.30; Figure 5c, left). In contrast, no correlation or trend for correlation between neuronal activation and behavior was found for Network 2 (R2=0.002, p=0.87), and the activity of this network was not affected by ADX71441 treatment (F1,15=0.40, p=0.53; η2=0.02; Figure 5c, right).
Stress-induced neuronal activity in an interconnected network of brain structures is strongly correlated with stress-induced relapse-like behavior, and is suppressed by ADX71441. Principal component extraction followed by varimax normalized rotation of c-Fos data identified two independent networks that showed high measures of within-network connectivity. Network 1 consisted of the NAcSh, DRN and mPFC, and its activity was highly correlated with relapse-like behavior (a). In contrast, activity of Network 2, which consisted of BLA, CeA and BNST did not show any correlation with behavior (b). Activity of Network 1, but not Network 2, was suppressed by ADX71441 (c; ***p<.0001; for detailed statistics, see Results section).
In summary, this analysis suggests that the ability of ADX71441 to prevent stress-induced relapse is related to its inhibition of a stress-responsive network that includes the DRN, NAcSh and mPFC. Of note, although activation of the CeA was suppressed by ADX71441 when analyzed individually, this structure did not appear to be part of the full network influenced by treatment. This conclusion is further supported by the observation that c-Fos activity in CeA did not correlate with reinstatement behavior (Supplementary Figure 4d).
Discussion
We report that ADX71441, a novel PAM with high selectivity for GABAB receptors, dose-dependently suppressed alcohol self-administration. This effect was robust, was evident in non-dependent rats at a dose of 3 mg/kg, and reached a >80% reduction of self-administration at a dose of 10 mg/kg. These findings are in line with prior results obtained with orthosteric GABAB receptor agonists, several of which have shown efficacy in attenuating responding for alcohol (Anstrom et al, 2003; Janak and Michael Gill, 2003; Liang et al, 2006; Maccioni et al, 2005; Walker and Koob, 2007). Except for the highest dose of 30 mg/kg, suppression of responding for alcohol was not the result of non-specific sedative or otherwise performance-impairing properties of the drug, as ADX71441 did not impair locomotor activity at doses between 1 and 10 mg/kg. An improved separation between suppression of alcohol self-administration and sedation is important, as a limitation of GABAB receptor agonists in treating alcohol use disorders is a narrow therapeutic window. Here, we found that ADX71441 displays a 10-fold separation between specific behavioral effects and sedation, and therefore may have a better therapeutic index than orthosteric GABAB receptors agonists for treatment of alcoholism. ADX71441 also decreased self-administration of saccharin, a non-caloric sweetener, indicating that GABAB PAM:s may decrease the acute reinforcing properties of multiple rewarding substances rather than modulating the reward from the caloric value of liquid reinforcers such as alcohol. In accordance with this observation, baclofen was shown to dose-dependently reduce responding for sucrose (Anstrom et al, 2003) and palatable food intake (Avena et al, 2014). Several brain regions including the lateral hypothalamus, nucleus accumbens (both core and shell), BNST have been shown to be activated following sucrose self-administration (Figlewicz et al, 2011) and further studies will be needed to identify the neural substrates through which ADX71441 act to prevent self-administration of sweet reinforcers.
A key finding of our study that further supports a utility of ADX71441 as a therapeutic in alcoholism is that rats with a history of dependence showed increased sensitivity to suppressions of alcohol self-administration by this compound. While the dose of ADX71441 required to significantly affect responding for alcohol in non-dependent animals was 3 mg/kg, a dose of 1 mg/kg was sufficient in animals with a history of dependence. This was observed in protracted abstinence, when symptoms of acute withdrawal are no longer present, and is therefore likely to reflect actions of ADX71441 on persistent neuroadaptations known to occur following a history of dependence (Heilig et al, 2010; Meinhardt et al, 2013). The sensitized response to potentiation of GABAB signaling observed in post-dependent animals may explain the discrepant findings in two major RCT investigating the efficacy of the orthosteric GABAB agonist baclofen to treat alcohol use disorder. An ambitious RCT that recruited severely alcohol-dependent patients with liver disease found that baclofen more than doubled the proportion of participants who achieved abstinence and maintained it for the 12 week duration of the study (Addolorato et al, 2007). On the other hand, an RCT conducted in the US that recruited less severely alcohol-dependent patients did not find that baclofen was superior to placebo (Garbutt et al, 2010). Our preclinical data support the notion that targeting GABAB receptors may be a particularly effective mechanism for treatment of severe alcohol addiction.
An objective of our investigation was to identify the neural substrates through which ADX71441 suppresses stress-induced relapse to alcohol seeking, in hope of facilitating the development of imaging based translational biomarkers for early clinical studies. We carried out a c-Fos mapping focused a priori on a network of brain structures previously shown to be associated with stress-induced relapse to alcohol seeking (Funk et al, 2006). We did not examine the activation of these structures per se, in comparison to an extinction baseline. To carry out an unbiased search for stress-activated structures whose activity is modulated by ADX71441, a complete design using a 2 × 2 factorial with footshock vs no footshock × ADX71441 vs vehicle would have been needed (Dayas et al, 2007; Zhao et al, 2006). In our view, this is unlikely to be a significant limitation, because an activation of these structures in the context of footshock stress-induced relapse to alcohol seeking has been repeatedly demonstrated both by others (Funk et al, 2006), and by our own laboratory (Schank et al, 2015), therefore allowing us to limit our analysis to reinstatement data. Using this analysis, we found that neuronal activity was attenuated by the GABAB PAM in the NAcSh, CeA and DRN, with a trend also in the mPFC. Surprisingly, c-Fos activation in two other structures implicated in stress-induced reinstatement by prior mapping efforts, BLA and BNST (Funk et al, 2006; Schank et al, 2015), was unaffected by ADX71441 administration.
A network analysis identified a network consisting of the DRN, NAcSh and mPFC within which activity was strongly correlated. Activity of this network accounted for more than 70% of the variance in reinstatement responding, and was robustly suppressed by ADX71441, making this network a plausible candidate neural substrate of ADX71441 activity to suppress stress-related relapse. This is further supported by the observation that the neural signature of ADX71441 was very similar to that recently found by our laboratory for another candidate medication that potently blocks stress-induced reinstatement, the neurokinin 1 (NK-1) receptor antagonist L822429 (Schank et al, 2015). Both these data-sets are consistent with observations that the mPFC sends direct projections to the NAcSh (Sesack et al, 1989), and that these projections play a role in reinstatement of alcohol (Barbier et al, 2016; Meinhardt et al, 2013) as well as heroin seeking (Bossert et al, 2012). Overall, the high correlation of activity within the DRN–NAcSh–mPFC network with behavior, and its selective suppression by ADX71441 provide evidence suggestive of a mechanistic role of this network for stress-induced relapse. Studies directly manipulating the activity of this network will be needed to positively establish this role. Meanwhile, however, identification of this network is of value per se in that it paves the way for developing translational biomarkers. Specifically, the ability of an ADX71441 dose to suppress fMRI activity of these structures following exposure to stressors that induce alcohol craving in humans would support target engagement by that dose.
In contrast to the DRN—NAcSh—mPFC network, the structures within which neuronal activation was unaffected by ADX71441, BLA and BNST, were found to be elements of a second stress-activated network identified by our analysis, whose activity did not correlate with relapse behavior. Surprisingly, CeA, whose activity was robustly suppressed by ADX71441, was also an element of network 2. Both the CeA and the NAcSh have been suggested to be part of an extended amygdala circuit that mediates stress-triggered relapse to drug seeking (Kalivas and Volkow, 2005), but the vast majority of studies on which this notion is based has been carried out with drugs other than alcohol. CeA activity was significantly suppressed by ADX71441, but did not correlate with relapse behavior, somewhat surprisingly suggesting that the activity of this structure may not be directly causally related to relapse to alcohol seeking.
An unexpected feature of ADX71441’s activity profile was its ability to potently suppress both stress- and cue-induced relapse to alcohol seeking. This suggests that ADX71441 may act either on both the distinct, but converging networks that mediate these two behaviors, or on a common final pathways that subserves drug seeking initiated by stress, drug cues or drug priming (Kalivas and Volkow, 2005). It has been shown that a series of projections from the prefrontal cortex to the NAc core to the ventral pallidum represents a final common pathway for drug seeking, independently of the initiating stimulus. In contrast, the extended amygdala subcircuit has been reported to specifically mediate drug seeking initiated by a stressor, while projections from the ventral tegmental area to the BLA are specifically involved in cue-induced drug seeking. Although not examined in the present study, it can be hypothesized that NAc Core is a structure through which the GABAB PAM may suppress cue-induced relapse to alcohol seeking. The NAc core receives major inputs from the prefrontal cortex (Kalivas and Volkow, 2005; Shirayama and Chaki, 2006), which we found in the present investigation potentially involved in mediating stress-induced relapse to alcohol seeking. Furthermore, ~95% of neurons in the NAc core are GABAergic medium spiny neurons, and GABAB receptors are abundant in this region (Shirayama and Chaki, 2006). The present study was primarily focused on examining the hypothesis that ADX71441 would suppress stress-induced relapse, and additional experiments will be required to map out the neural substrates through which it blocks cue-induced relapse.
In conclusion, our data extend previous findings indicating that targeting GABAB receptors may be beneficial in treating drug addiction, and that positive allosteric modulators of GABAB receptors, by retaining the efficacy of GABAB receptor agonists and improving upon their side effect profile, should be considered for alcohol use disorder treatment in humans. We also provide preclinical data indicating that maximal efficacy of ADX71441 can be expected in clinical trials that enrich for severely dependent patients. Finally, we provide a basis for developing an fMRI based translational biomarker that has the potential to advance clinical development.
Funding and disclosure
This work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism and funding from the Swedish Research Council. The remaining authors declare no conflict of interest.
References
Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L et al (2002a). Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol 37: 504–508.
Addolorato G, Caputo F, Capristo E, Janiri L, Bernardi M, Agabio R et al (2002b). Rapid suppression of alcohol withdrawal syndrome by baclofen. Am J Med 112: 226–229.
Addolorato G, Leggio L, Cardone S, Ferrulli A, Gasbarrini G (2009). Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol 43: 559–563.
Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A et al (2007). Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet 370: 1915–1922.
Anstrom KK, Cromwell HC, Markowski T, Woodward DJ (2003). Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res 27: 900–908.
Augier E, Dulman RS, Rauffenbart C, Augier G, Cross AJ, Heilig M (2016). The mGluR2 positive allosteric modulator, AZD8529 and cue-induced relapse to alcohol seeking in rats. Neuropsychopharmacology 41: 2932–2940.
Augier E, Dulman RS, Singley E, Heilig M (2017). A method for evaluating the reinforcing properties of ethanol in rats without water deprivation, saccharin fading or extended access training. J Vis Exp 119: e53305.
Augier E, Flanigan M, Dulman RS, Pincus A, Schank JR, Rice KC et al (2014). Wistar rats acquire and maintain self-administration of 20% ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology (Berl) 231: 4561–4568.
Avena NM, Bocarsly ME, Murray S, Gold MS (2014). Effects of baclofen and naltrexone, alone and in combination, on the consumption of palatable food in male rats. Exp Clin Psychopharmacol 22: 460–467..
Barbier E, Johnstone AL, Khomtchouk BB, Tapocik JD, Pitcairn C, Rehman F et al (2016). Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol Psychiatry (doi:10.1038/mp.2016.131; e-pub ahead of print).
Besheer J, Lepoutre V, Hodge CW (2004). GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6 J mice. Psychopharmacology (Berl) 174: 358–366.
Bormann J (2000). The 'ABC' of GABA receptors. Trends Pharmacol Sci 21: 16–19.
Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M et al (2012). Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32: 4982–4991.
Brebner K, Childress AR, Roberts DC (2002). A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol 37: 478–484.
Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G et al (2002). The GABA(B) receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcohol 37: 499–503.
Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL (2003). Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology (Berl) 167: 221–224.
Conn PJ, Christopoulos A, Lindsley CW (2009). Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8: 41–54.
Cott J, Carlsson A, Engel J, Lindqvist M (1976). Suppression of ethanol-induced locomotor stimulation by GABA-like drugs. Naunyn Schmiedebergs Arch Pharmacol 295: 203–209.
Cousins MS, Roberts DC, de Wit H (2002). GABA(B) receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend 65: 209–220.
Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F (1987). GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol 4: 469–472.
Dayas CV, Liu X, Simms JA, Weiss F (2007). Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry 61: 979–989.
Dutar P, Nicoll RA (1988). A physiological role for GABAB receptors in the central nervous system. Nature 332: 156–158.
Epstein DH, Preston KL, Stewart J, Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189: 1–16.
Fadda P, Scherma M, Fresu A, Collu M, Fratta W (2003). Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse 50: 1–6.
Figlewicz DP, Bennett-Jay JL, Kittleson S, Sipols AJ, Zavosh A (2011). Sucrose self-administration and CNS activation in the rat. Am J Physiol Regul Integr Comp Physiol 300: R876–884..
Funk D, Li Z, Le AD (2006). Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience 138: 235–243.
Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA (2010). Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 34: 1849–1857.
Heilig M, Egli M (2006). Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111: 855–876.
Heilig M, Egli M, Crabbe JC, Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15: 169–184.
Heilig M, Sommer WH, Spanagel R (2016). The need for treatment responsive translational biomarkers in alcoholism research. Curr Top Behav Neurosci 28: 151–171.
Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944.
Hwa LS, Kalinichev M, Haddouk H, Poli S, Miczek KA (2014). Reduction of excessive alcohol drinking by a novel GABAB receptor positive allosteric modulator ADX71441 in mice. Psychopharmacology (Berl) 231: 333–343.
Janak PH, Michael Gill T (2003). Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 30: 1–7.
Kalinichev M, Palea S, Haddouk H, Royer-Urios I, Guilloteau V, Lluel P et al (2014). ADX71441, a novel, potent and selective positive allosteric modulator of the GABA(B) receptor, shows efficacy in rodent models of overactive bladder. Br J Pharmacol 171: 995–1006.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Leggio L, Garbutt JC, Addolorato G (2010). Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets 9: 33–44.
Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D et al (2006). The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology 50: 632–639.
Ling W, Shoptaw S, Majewska D (1998). Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology 18: 403–404.
Maccioni P, Serra S, Vacca G, Orru A, Pes D, Agabio R et al (2005). Baclofen-induced reduction of alcohol reinforcement in alcohol-preferring rats. Alcohol 36: 161–168.
Macey DJ, Schulteis G, Heinrichs SC, Koob GF (1996). Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol 13: 163–170.
May LT, Christopoulos A (2003). Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol 3: 551–556.
Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M et al (2013). Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci 33: 2794–2806.
Meinhardt MW, Sommer WH (2015). Postdependent state in rats as a model for medication development in alcoholism. Addict Biol 20: 1–21.
Perdona E, Costantini VJ, Tessari M, Martinelli P, Carignani C, Valerio E et al (2011). In vitro and in vivo characterization of the novel GABAB receptor positive allosteric modulator, 2-{1-[2-(4-chlorophenyl)-5-methylpyrazolo[1,5-a]pyrimidin-7-yl]-2-piperidinyl}eth anol (CMPPE). Neuropharmacology 61: 957–966.
Pin JP, Prezeau L (2007). Allosteric modulators of GABA(B) receptors: mechanism of action and therapeutic perspective. Curr Neuropharmacol 5: 195–201.
Rimondini R, Arlinde C, Sommer W, Heilig M (2002). Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J 16: 27–35.
Roberts DC, Andrews MM (1997). Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology (Berl) 131: 271–277.
Roberts DC, Andrews MM, Vickers GJ (1996). Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15: 417–423.
Rogers J, Wiener SG, Bloom FE (1979). Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27: 466–486.
Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE et al (2015). Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology 99: 106–114.
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290: 213–242.
Shirayama Y, Chaki S (2006). Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4: 277–291.
Sivilotti L, Nistri A (1991). GABA receptor mechanisms in the central nervous system. Prog Neurobiol 36: 35–92.
Urwyler S (2011). Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev 63: 59–126.
Walker BM, Koob GF (2007). The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31: 11–18.
Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26: 9967–9974.
Acknowledgements
We thank Gaëlle Augier for helpful comments on a previous draft of the manuscript and help with editing. We thank Addex Therapeutics for kindly providing ADX71441.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Augier, E., Dulman, R., Damadzic, R. et al. The GABAB Positive Allosteric Modulator ADX71441 Attenuates Alcohol Self-Administration and Relapse to Alcohol Seeking in Rats. Neuropsychopharmacol 42, 1789–1799 (2017). https://doi.org/10.1038/npp.2017.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.53